Introduction
Cognitive deficits make a significant contribution to disability in many psychotic disorders (Green et al. Reference Green, Kern, Braff and Mintz2000; Wykes & Reeder, Reference Wykes and Reeder2005). Often predating the emergence of clinical symptoms, these deficits persist throughout the illness, despite fluctuations in clinical symptom severity. Because current antipsychotic medications do not adequately treat these deficits (Green, Reference Green1996; Fett et al. Reference Fett, Viechtbauer, Penn, van Os and Krabbendam2011), behaviour-based therapies designed to remediate cognitive deficits, an approach known as cognitive remediation training (CR), have become a significant focus of research.
CR targets difficulties with cognitive skills such as attention, memory, problem-solving, information-processing-speed, organization, planning and social cognition. Despite significant differences between training programmes in both methods and administration, a meta-analysis by Wykes et al. (Reference Wykes, Huddy, Cellard, McGurk and Czobor2011) based on over 2000 participants found consistent evidence of cognitive gains associated with CR, yielding an average effect size of 0.45 across the range of interventions considered. Importantly, these benefits are not confined to cognition, but were also associated with benefits to social and occupational functioning (Wykes et al. Reference Wykes, Huddy, Cellard, McGurk and Czobor2011).
Several questions about CR remain, including the cost-effectiveness of the various approaches taken and their potential for integration into standard clinical care (Patel et al. Reference Patel, Knapp, Romeo, Reeder, Matthiasson, Everitt and Wykes2010). Even if the cost of CR compares favourably to currently used pharmacotherapy, the number of therapist hours involved are typically substantial, representing a potential challenge given the generally limited availability of psychosocial treatments for psychosis. Efforts to address this issue have included group-based training (Lilienthal et al. Reference Lilienthal, Tamez, Shelton, Myerson and Hale2013) and, recently, computer-based training using software that allows task difficulty to be automatically varied according to changes in patients’ performance. While having the potential to limit the need for 1:1 support for each session, an important question for such ‘e-health’ initiatives is to determine patients’ capacity to carry out such ‘remote’ training and how much training support is required to adequately facilitate participation.
In a preliminary ‘proof of concept’ study, we recently investigated whether patients benefitted from one such remote training programme in terms of improved memory function. This training programme is specifically focused on training working memory (WM) – the ability to ability to maintain and manipulate information over a period of seconds – following a hypothesis that WM training benefits cognitive function more generally, and may potentially achieve these gains more efficiently than programmes with a more general focus. In support of this hypothesis, training programmes that exclusively focused on WM in non-schizophrenia populations have been associated with a transfer of benefits to other cognitive functions, including attention, problem-solving and fluid intelligence (Jaeggi et al. Reference Jaeggi, Buschkuehl, Jonides and Perrig2008; Jaeggi et al. Reference Jaeggi, Buschkuehl, Perrig and Meier2010; Lilienthal et al. Reference Lilienthal, Tamez, Shelton, Myerson and Hale2013; Rudebeck et al. Reference Rudebeck, Bor, Ormond, O'Reilly and Lee2012; Salminen et al. Reference Salminen, Strobach and Schubert2012; Kundu et al. Reference Kundu, Sutterer, Emrich and Postle2013). In psychosis, only a few studies have exclusively targeted WM training, but with promising results (Wexler et al. Reference Wexler, Anderson, Fulbright and Gore2000; Haut et al. Reference Haut, Lim and MacDonald2010; Hubacher et al. Reference Hubacher, Weiland, Calabrese, Stoppe, Stöcklin, Fischer-Barnicol, Opwis and Penner2013; Subramaniam et al. Reference Subramaniam, Luks, Garrett, Chung, Fisher, Nagarajan and Vinogradov2014). Of these, the study by Subramanian further reported that WM training-related cortical changes were associated improved occupational functioning. In our proof of concept study, patients who trained remotely and received only 30–60 min of support per week from a therapist showed significant benefits in memory performance compared with patients receiving treatment as usual (Hargreaves et al. Reference Hargreaves, Dillon, Anderson-Schmidt, Corvin, Fitzmaurice, Castorina, Robertson and Donohoe2015).
Improved cognitive performance has repeatedly been associated with changes in cortical structure and function. Reviews of WM-based intervention in particular (Klingberg, Reference Klingberg2010; Constantinidis & Klingberg, Reference Constantinidis and Klingberg2016) suggest that WM training is associated with changes in brain activity in frontal and parietal cortex and basal ganglia, as well as changes in dopamine receptor density. Klingberg further suggests that observed transfer of the training effects to non-trained WM tasks is consistent with the notion of training-induced plasticity in a common neural network for WM. While evidence supporting training-induced changes in cortical activity is widespread in non-psychosis samples (Constantinidis & Klingberg, Reference Constantinidis and Klingberg2016), evidence of similar training-related changes in schizophrenia is only beginning to be reported (Li et al. Reference Li, Xiao, Zhao, Leung, Cheung and Chan2015).
The aim of this study (registered at ClinicalTrials.gov with ID: NCT01903707) was to investigate the effectiveness of this low support remotely accessed computerised WM training programme in a single blind randomised controlled trial (RCT) of patients with psychosis. Following on our proof of concept study (Hargreaves et al. Reference Hargreaves, Dillon, Anderson-Schmidt, Corvin, Fitzmaurice, Castorina, Robertson and Donohoe2015), we hypothesised that, compared to an active control condition, WM training would result in cognitive improvements in both (untrained) WM task performance, and performance on other memory tasks (e.g. verbal episodic memory). We also hypothesised, based on the studies cited above, that this training, if successful, would result in improved general cognitive function (based on measures of general intelligence), and in improved social and occupational function. We further hypothesised that any improvements observed would be associated with increased neural response during WM task performance using functional MRI (fMRI). Finally, given the evidence that resting state functional connectivity correlates with WM capacity (Stevens et al. Reference Stevens, Tappon, Garg and Fair2012), and strengthens the fronto-parietal network following training (Jolles et al. Reference Jolles, van Buchem, Crone and Rombouts2013; Takeuchi et al. Reference Takeuchi, Taki, Nouchi, Hashizume, Sekiguchi, Kotozaki, Nakagawa, Miyauchi, Sassa and Kawashima2013), we tested the hypothesis that CR training would result in the same functional connectivity in this group also.
Methods
Participants
Following ethical approval for the study, 90 participants were recruited from community health teams from various clinical services in Dublin, Wicklow and Galway, and through the Dublin branch of the National Learning Network, a community-based rehabilitation service. Patients were referred by their local treatment or rehabilitation teams following a series of presentations made about CR by the study team. Diagnosis was confirmed by a trained psychiatric research nurse based on a Structured Clinical Interview for DSM-IV (SCID) and review of all available information – including family or staff report, and chart review. Criteria for inclusion in the study were that participants were aged between 18 and 65 years, had a history of psychosis, were community-based and clinically stable (in the opinion of the treating team), and were engaged in some activity (e.g. part time work, attending a rehabilitation clinic for at least 2 days each week). Exclusion criteria included a history of organic impairment, head injury resulting in loss of consciousness, or drug abuse in the preceding 3 months.
Following study approval from relevant ethics boards, participants were enrolled after giving informed written consent. Participants did not receive compensation for participation in the study, although travel costs related to MRI participation were reimbursed. While enrolled, in addition to CR/Control treatment, all participants continued to receive treatment as usual (clinical review and medication) and no patient (to our knowledge) received other psychological-based treatments while enrolled in the study.
Randomisation procedure for group allocation and blinding
A randomisation table, based on a stratified block sampling procedure based on age (over and under 40 years old) and gender, was created by an independent statistician and administered by the CR therapist following baseline assessment. Only the CR therapist and participants were aware of group allocation and extensive steps were taken to maintain blinding. Communication about the group allocation of participants was not allowed, separate offices were used by the CR therapist and assessors in addition to separate storage and management of data. Similarly, participants were instructed to not divulge their group allocation to the follow-up assessor. At the end of the study, assessors were asked to guess the treatment allocation. The probability of assessors guessing the correct group was approximately 50%, suggesting successful blinding. Trial design and primary outcome measures did not differ from those originally registered.
Figure 1 shows the study consort diagram. Of the 85 patients who participated in either the treatment arm or control arm, data for 55 (64.7%) were available at 2-week post-treatment follow-up, and 32 (37.6%) at 3–6-month post-treatment follow-up.
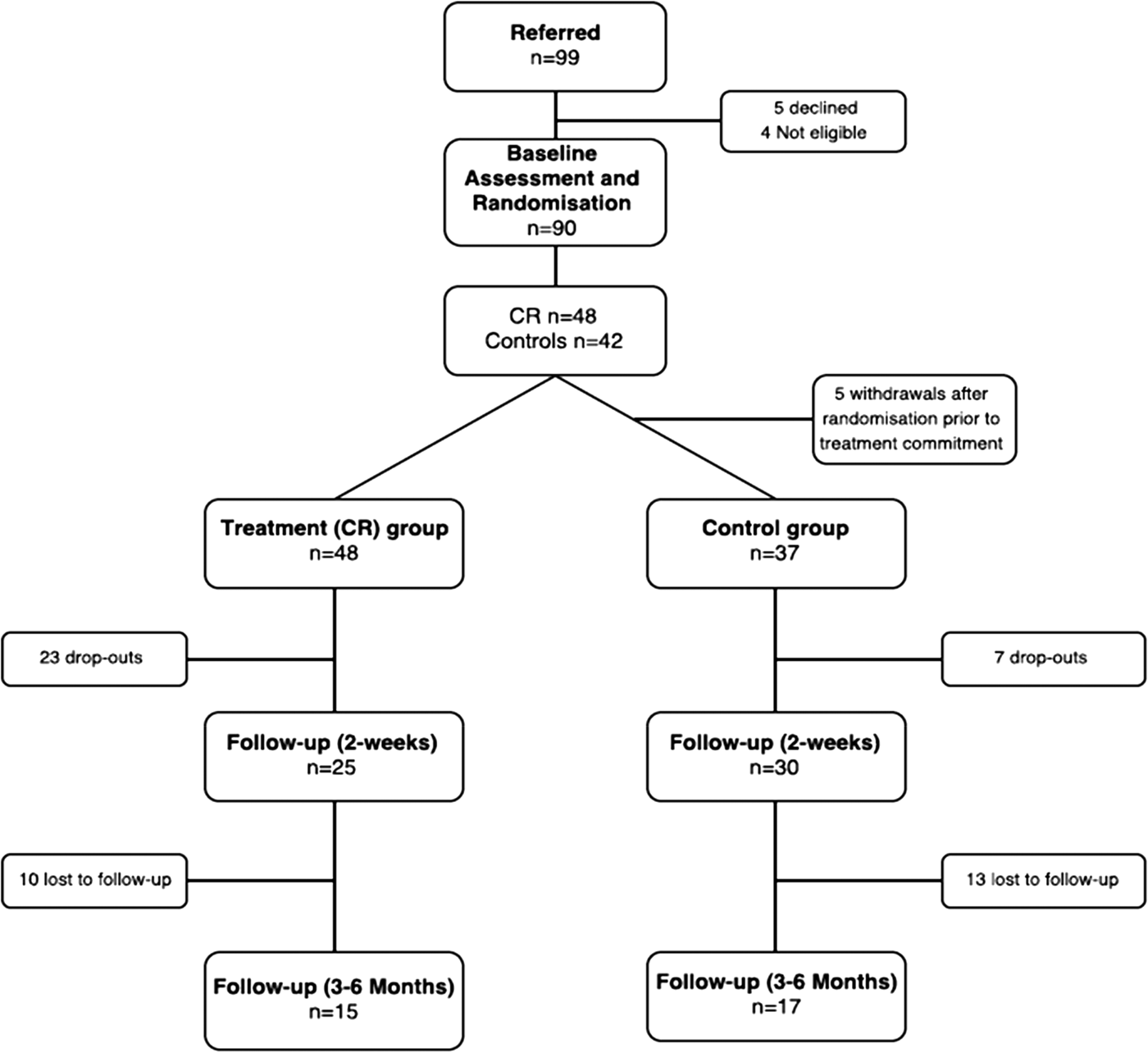
Fig. 1. Consort diagram. Of the 85 patients who participated in either the treatment arm or control arm, data for 55 (64.7%) was available at 2-week post-treatment follow-up, and 32 (37.6%) at 3–6-month post-treatment follow-up.
CR training
An online CR training programme, that was developed by us and specifically targeted WM, was used in the study (McAvinue et al. Reference McAvinue, Golemme, Castorina, Tatti, Pigni, Salomone, Brennan and Robertson2013; Hargreaves et al. Reference Hargreaves, Dillon, Anderson-Schmidt, Corvin, Fitzmaurice, Castorina, Robertson and Donohoe2015). Prior to commencing training, computer access and training needs (for software usage) of participants were evaluated. If the participants did not have internet access, a laptop and internet dongle were provided for the duration of training, as was any training required with accessing and logging on to the training website (approximately half of those who completed the programme).
The programme, which was web-based, targeted both auditory and visual WM modalities following Baddeley's (Reference Baddeley2000) model. The programme was an 8-week programme participants were required to complete within a 12-week window and consisted of: (a) psycho-education on the nature of WM, (b) strategy-based learning and (c) practice of nine WM focused training exercises that were gradually introduced over a 5-week period, beginning with the easier exercises first. Participants were required to complete 30–40 min of training a day, 5 days a week. The nine WM training tasks, which consisted of n-back tasks and classic digit span tasks were designed to be, to at least some extent, ecologically valid by relating training to every-day tasks. To achieve this, a context was presented for each task; for example, on the n-back faces task, the context given was that the participant was at a party and being introduced to various individuals and had to remember the last four faces they saw, etc. Task difficulty level was automatically adjusted in terms of the speed and amount of information presented according to patients’ progress in training, based on the criteria of achieving an accuracy 80% on a previous difficulty level. At the end of each session participants were given visual feedback via a graph of time in training and scores obtained, so that they could track their individual progress. Participants met with the study therapist once per week for the 8 weeks of the training for 45 min. The sessions with the therapist following a structured motivational interviewing approach to support training, and focused on: (a) the applicability of training to real-life situations, (b) use of WM strategies in daily activities (e.g. chunking to remember telephone numbers, use of a ‘mental blackboard’), (c) reinforcement of the generalisability of WM training by reviewing how participants had employed the training in daily life in the preceding week and ways of increasing this in the following week, and (d) problem solving any difficulties encountered using the programme. Usage of the programme in terms of minutes and hours of exercises complete was monitored online by the therapist.
Active control condition
The control condition was designed to mirror the amount of time the participants spent with the therapist in the intervention group. As the first randomised trial of this low support training intervention, this approach was taken rather than including a sham training condition to enable us establish the full effects of the WM training intervention. In the first condition to Participants in the control group met weekly with the same CR therapist as the intervention condition and for the same amount of time (on average 45 min in both groups) and were encouraged to discuss topics of the participants’ choice in an open-ended conversation. Topics of conversation spanned from patients’ previous week's events to hobbies and current affairs. Participant symptoms were not directly approached and there was no set agenda for each session apart from a weekly check-in to establish the participants’ well-being. Notes pertaining to each session's content were recorded and the therapist aimed to be non-directive and empathetic in approach.
Clinical and neuropsychological assessment
In addition to completing a SCID assessment with either a clinical research nurse or postdoctoral level psychologist, all patients completed the schedule for the assessment of positive symptoms and the schedule for the assessment of negative symptoms (SAPS and SANS; Andreasen, Reference Andreasen1984, Reference Andreasen1989) and gave details regarding age at illness onset, number of previous hospital admissions and current medication dosage, which was converted into chlorpromazine equivalents. After this information was collected, all patients completed the following measures of neuropsychological, social functional and well-being both prior to training (described as baseline data) and within 2 weeks of completing training (described as 2-week post-training). Neuropsychological measures were also then readministered 3–6 months later.
Primary outcome measures were included as follows
Episodic memory was assessed using the logical memory subtest, immediate and delayed conditions, from the Wechsler Memory Scale, 3rd edition (Wechsler, Reference Wechsler1998).
WM; verbal and spatial memory was assessed using letter number sequencing (LNS) from WMS-III and the spatial WM (SWM) from The Cambridge Neuropsychological Test Automated Battery (Robbins et al. Reference Robbins, James, Owen, Sahakian, McInnes and Rabbitt1994).
Secondary outcome measures were included as follows
General cognitive ability was measured using the similarities and matrix reasoning subtests from the Wechsler abbreviated scale of Intelligence (WASI; Wechsler, Reference Wechsler1999), which is a brief, reliable measure of cognitive ability routinely used in clinical, educational and research settings. From these two subtests, a measure of full scale IQ was derived based on the published norms available for the two subtest version of the test.
Executive functioning was measured using the CANTAB Stockings of Cambridge task (SOC), the computerised Wisconsin Card Sorting Task (Wisconsin Card Sorting Test® Computer Version 4) (Heaton & Staff, Reference Heaton and Staff2003), the STROOP (Stroop, Reference Stroop1935) and the Trail making test (TMT) (Reitan & Wolfson, Reference Reitan and Wolfson1985).
Social cognition was measured using the total scores from the Reading the mind in the eyes task (Baron-Cohen et al. Reference Baron-Cohen, Jolliffe, Mortimore and Robertson1997), a measure of mental state decoding, which is an aspect of theory of mind ability.
Social & Functional outcome were measured using three scales: (a) The total score from the Social and Occupational Functioning Assessment Scale (Rybarczyk, Reference Rybarczyk, Kreutzer, DeLuca and Caplan2011), which provides a rating of global social and occupational function independent of clinical symptoms, (b) The total score from the UCSD performance-based skills assessment – brief (Mausbach et al. Reference Mausbach, Tiznado, Cardenas, Jeste and Patterson2016), which provides a performance-based skills assessment focusing on finances and communication, and (c) the problem-solving subscales from the Independent Living Scales (35), which rates level of independence in managing everyday situations requiring problem-solving skills. Only this subscale of the test was administered to limit the total length of the assessment battery.
Measures of well-being were measured using the Rosenberg self-esteem questionnaire (Rosenberg, Reference Rosenberg1965), and the World Health Organisation Quality of Life Project (WHO, 1996).
Statistical analysis
Neuropsychological data analysis
All data were processed using Analysis of Covariance (ANCOVA) carried out in SPSS version 22. For both outcome time-points [i.e. (a) 0–2 weeks post-treatment and (b) 3–6-month post-treatment], a series of ANCOVAs were carried out in which performance on each task was entered as the dependent variable (see Table 2 for full list of measures), group (CR v. control) as the independent variable, and baseline performance on the dependent variable was entered as a covariate. Gender and age also served as covariates.
MRI methods
The fMRI n-back sample included 15 CR patients and 15 control patients. The fMRI resting-state sample included 14 CR patients and 15 control patients. All fMRI n-back and resting-state samples were chosen from the larger patient sample, based on whether they consented to participate in MRI scanning and were able to complete MRI scans across two timepoints. One n-back participant and two resting-state participants were excluded due to excessive fMRI signal dropout, leaving a final sample of 29 n-back participants and 27 resting-state participants.
MRI data were acquired using a 3T Philips Achieva MR system using standard methods (described in online Supplemental material). We used a 2-back task to examine WM-dependent changes in neural activity [blood oxygenation level-dependent (BOLD) signal] (38). We also acquired fMRI data during a resting-state scan (rs-fMRI), during which participants kept their fixation on a cross presented on a screen for 7 min.
For both 2-back and resting-state data, preprocessing was performed using Statistical Parametric Mapping (SPM8, v6313) and MATLAB R2014a (v8.3.0.532) including realignment to the mean image, normalisation to MNI (Montreal Neurological Institute) space with a voxel size of 2 × 2 × 2 mm3 and smoothing with an 8 mm FWHM (full-width at half-maximum) isotropic Gaussian filter. The Artefact Detection Tools (ART) toolbox was used for artefact detection.
The general linear model was used to perform statistical analysis of 2-back data with a contrast of 2-back > 0-back. Contrast maps were then entered into a flexible factorial model to examine group × time interactions with factors subject (variance set to equal, independence set to yes), group (variance set to unequal, independence set to yes) and time (variance set to equal, independence set to no) (Gläscher & Gitelman, Reference Gläscher and Gitelman2008).
Rs-fMRI pre-processing and statistical analysis followed the same methods previously used by our group (McCarthy et al. Reference McCarthy, Skokauskas, Mulligan, Donohoe, Mullins, Kelly, Johnson, Fagan, Gill, Meaney and Frodl2013; Mothersill et al. Reference Mothersill, Tangney, Morris, McCarthy, Frodl, Gill, Corvin and Donohoe2016), including functional connectivity analysis using the CONN toolbox (v15; National Institutes of Health Blueprint for Neuroscience Research). Functional connectivity maps generated by this analysis were entered into a flexible factorial model, as with the 2-back analysis. Based on a previous rs-fMRI study from our group (Mothersill et al. Reference Mothersill, Tangney, Morris, McCarthy, Frodl, Gill, Corvin and Donohoe2016), we examined functional connectivity within the default network, affective network, and ventral attention network, as we previously identified significant differences in functional connectivity of these networks in patients with schizophrenia compared with healthy controls. In addition, we examined the cognitive control network due to the putative importance of this network in WM training. A more detailed MRI methodology is presented in the online Supplementary material.
Results
A total of 90 patients were recruited into the study, as illustrated in Fig. 1 (Consort diagram). Of 48 and 42 participants randomised to the intervention and control conditions respectively, no differences were observed between groups in either age, gender or years in education (see Table 1). Clinically, no differences were observed between groups at baseline in terms of diagnosis, age at onset, duration of illness, symptom severity or current antipsychotic medication dosage (measured in chlorpromazine equivalents).
Table 1. Demographic and clinical characteristics of the intervention and control groups

Neuropsychological effects of CR
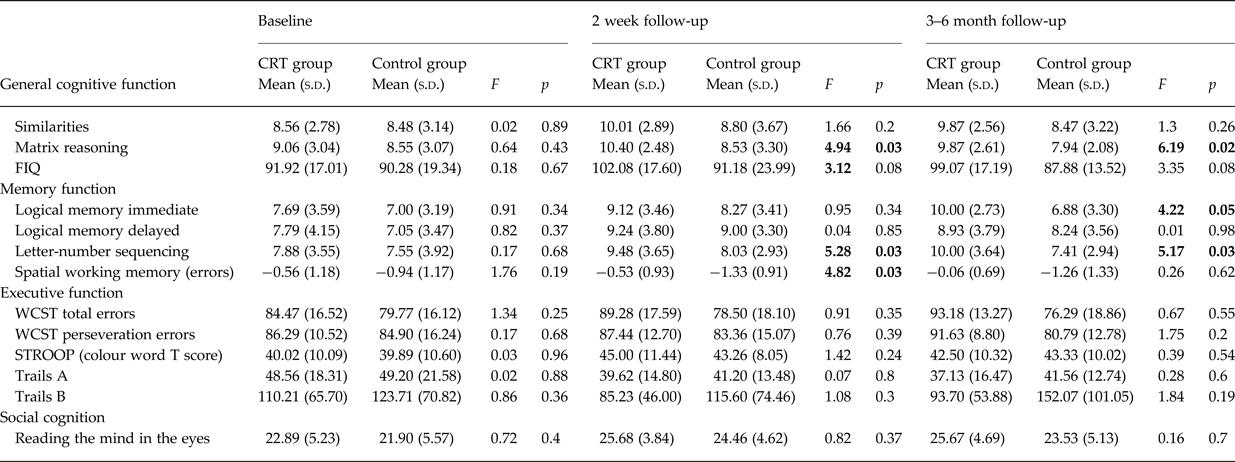
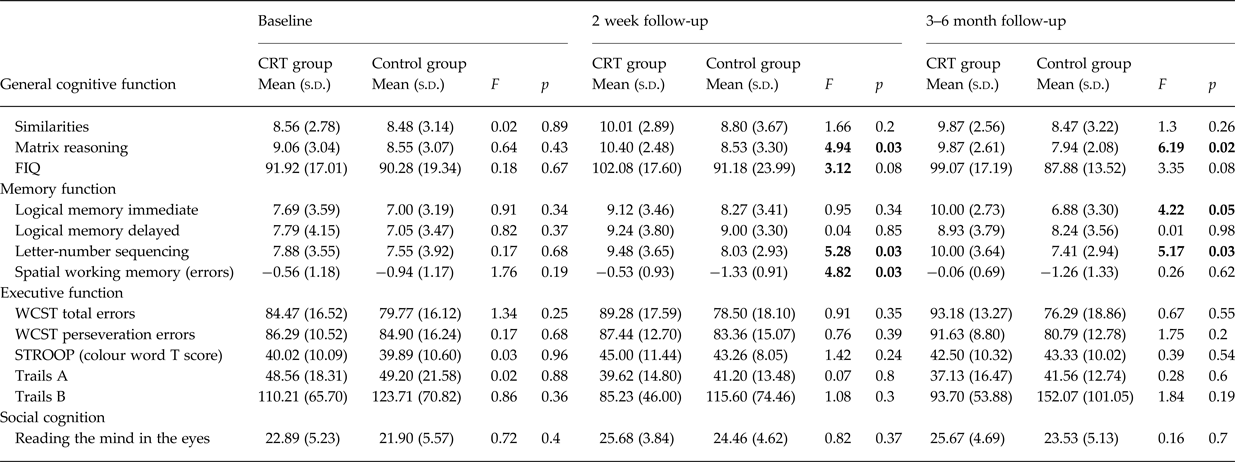
Mean scores for the intervention group and the control group for all cognitive variables analysed at baseline, 2 weeks post-treatment, and 3–6-month follow-up are presented in Table 2. At baseline (pre-treatment), no differences between the intervention group and the control group were observed for any cognitive measure (all p > 0.05).
Table 2. Comparison of cognitive performance and social function between the intervention and control groups at baseline, 2 weeks post-treatment, and 3–6 months post-treatment
SOFAS, Social and occupational functional assessment scale; UPSA-B, UCSD performance-based skills assessment brief; ILS-PS, Independent living scale, problem-solving scale.
For the 3–6 months follow-up period, the average length of time since training was 5 months.
Values in bold indicate significance p < 0.05.
After co-varying for baseline, significant differences in 2-week post-treatment follow-up scores were observed between the groups for both verbal and spatial WM task performance, and for matrix reasoning/performance IQ. A trend level difference was also observed for full scale IQ. On each of these measures, the intervention group significantly outperformed the control group. No other differences in cognitive performance were observed.
Mean differences between the intervention and control group at 2 weeks post-treatment follow-up remained significant at the 3–6-month follow-up, with two differences (see Fig. 2). Firstly, for spatial WM, although the mean differences between groups mirrors that seen at 2 weeks post-treatment follow-up (the intervention group showing near normal spatial WM performance of −0.05), this difference is no longer statistically significant. Secondly, significant differences were observed in verbal episodic memory between groups, such that the intervention group significantly outperformed the control group at this timepoint.
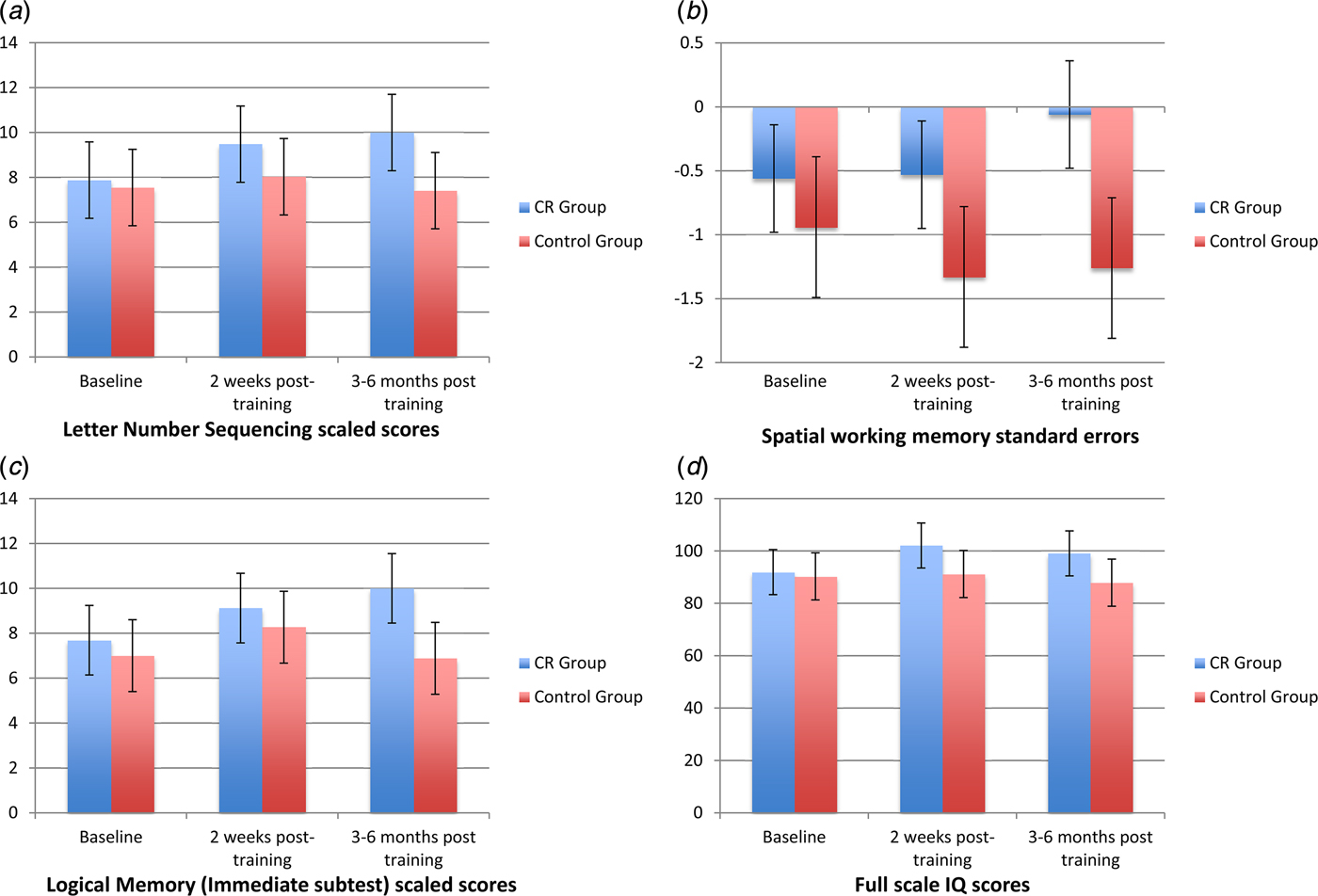
Fig. 2. Cognitive performance at baseline (pre-treatment), 2 weeks post-treatment, and 3–6 months follow-up for measures on which significant cognitive performance differences were observed: (a): Verbal working memory, (b) Spatial working memory, (c) Verbal episodic memory, and (d) Performance IQ.
Effects of CR on social and occupational function, self-esteem and quality of life
No differences in self-esteem (Rosenberg Self Esteem Inventory) were observed at either 2 weeks post-intervention or 3–6 months post-intervention follow-up assessment timepoints (see Table 3). Similarly, no differences in quality of life, (WHOQOL), were observed at either timepoint. In contrast, differences in social and occupational function were observed at both timepoints. At 2 weeks post-treatment follow-up, the intervention group had significantly better total scores on the UPSA-B than the control group. In a post hoc analysis these differences appear to have been driven by the financial subtest (F = 12.56; p = 0.001) as the communication scores did not show a significant change (p > 0.05). While significant differences on the financial subtest remained at the 3–6 months follow-up period (F = 4.48; p = 0.045), total score differences were no longer significant. Similarly, patients in the CR group showed trend level improvements in SOFAS scores at 2-week post-treatment follow-up, benefits which were statistically significant at 3–6-month follow-up. Finally, the CR group at 3–6 months significantly outperformed the control group on our ILS problem-solving subtest at both follow-up time periods.
Table 3. Differences between CR and Control Group in social and occupational function at baseline, 2 weeks post-treatment, and 3–6-month post-treatment

Data: Mean (s.d.). SOFAS, Social and occupational functional assessment scale; UPSA-B, UCSD performance based skills assessment brief; ILS-PS, Independent living scale, problem-solving scale.
Values in bold indicate significance p < 0.05.
MRI results
N-back participant demographics
No significant differences were observed between the CR and control groups for age, gender, IQ, n-back WM accuracy or reaction time (n-back 2-back condition) at baseline (all p > 0.05, see online Supplementary Table S2). At 2 weeks post-treatment follow-up, despite being underpowered, differences between groups in n-back performance were observed, such that CR patients had significantly slower reaction times and showed trend level (p = 0.052) improvements in accuracy during the task (see online Supplementary Table S3). There were no significant time × group interactions observed on number of ART outliers estimated for the n-back task across time 0 and time 1, across CR and control patient groups (p > 0.05, see online Supplementary Table 2).
Resting-state participant demographics
Due to the potentially confounding effects of motion on the estimation of functional connectivity, mean scan to scan translation and rotation were calculated in MATLAB for each participant for each timepoint for the resting-state data. No significant time × group interactions were observed on mean translation, mean rotation, or number of ART outliers estimated for the resting-state fMRI data across time 0 and time 1, across CR and control patient groups (p > 0.05, see online Supplementary Table S4).
Effects of CR on BOLD response during N-back task
No significant group × time interactions were observed on BOLD response during the n-back task (contrast 2-back > 0-back).
Effects of CR on functional connectivity during rest
We examined interactions between group (CR or control) and time (pre- or post-intervention) on connectivity of our six seed regions of interest (see online Supplementary Table S1). Significance was initially set at p < 0.05, FWE-corrected. Overall, we observed two group × time effects.
A group × time effect was observed on connectivity between the right precuneus and the left inferior parietal lobule (t max = 6.53, n = 27; see online Supplementary Table S5 and Fig. 3). A second group × time effect was observed on connectivity between the left anterior cingulate and right midcingulate (t max = 4.62, n = 27). Given that we examined functional connectivity across four resting-state networks, we also examined group × time effects at a more conservative threshold of p < 0.0125, FWE-corrected (i.e. correcting for the four networks analysed). The precuneus finding survived this additional correction, but the anterior cingulate finding did not (group × time effects on precuneus connectivity are reported at the p < 0.0125 threshold in).
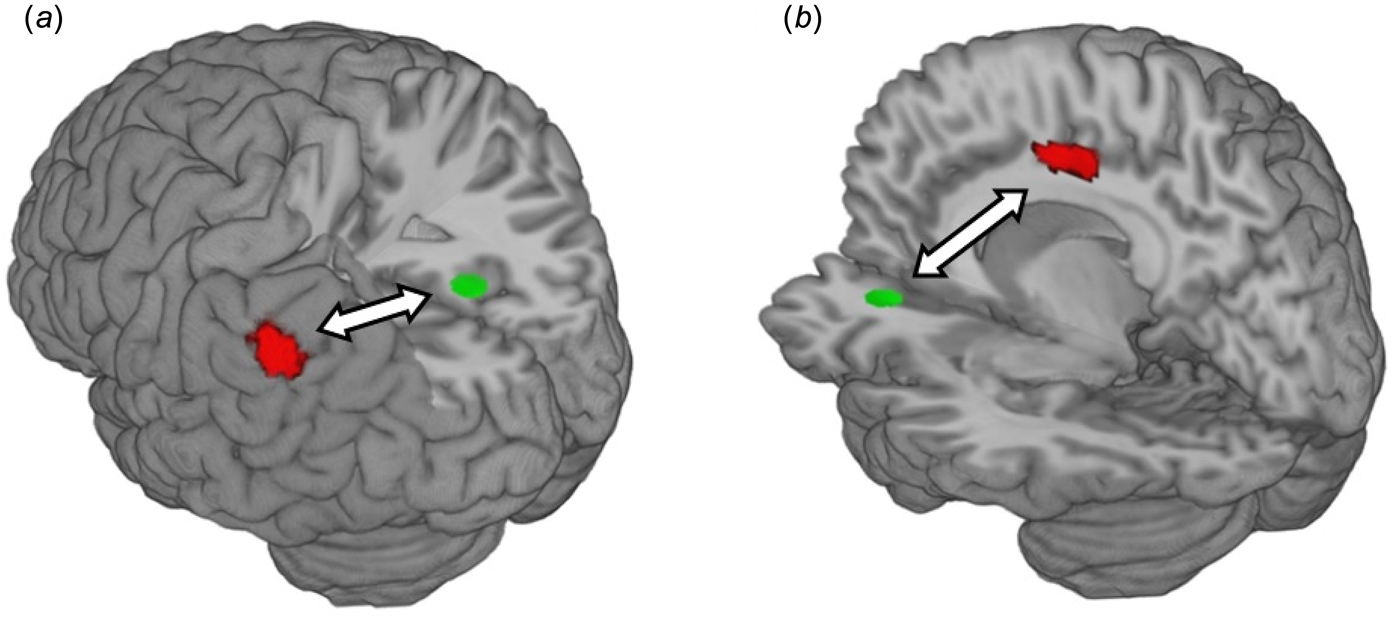
Fig. 3. CR group × time interaction on functional connectivity during rest. (a) CR group × time interaction on functional connectivity between the right precuneus (seed region in green) and the left inferior parietal lobule (cluster in red) (b) CR group × time interaction on functional connectivity between the left anterior cingulate cortex (seed region in green) and the right midcingulate cortex (cluster in red). Arrows represent functional connectivity; all clusters significant at p < 0.05, FWE-corrected across the whole brain at the cluster level. Clusters were rendered on the ‘ch256’ brain template using MRIcroGL (http://www.mccauslandcenter.sc.edu/mricrogl/).
Discussion
The study reports on the neuropsychological, functional, and neural effects of a low support CR programme for psychosis. While CR has received significant support in terms of its efficacy, the aim of the study was to ascertain whether patients could benefit from a programme involving only 1 h per week face-to-face contact with a clinician – the amount of contact often made available in psychological treatments. Using a RCT methodology involving stable community-based outpatients with psychosis, and a training programme that specifically targeted WM, three main outcomes were observed. Firstly, patients completing the CR training outperformed control group patients on measures of WM, episodic memory, and general cognitive ability, at 2 weeks post-treatment assessment and 3–6 months post-treatment assessment. Secondly, CR patients outperformed control patients on both skills-measured and rater-measured social and occupational function, again at both 2 weeks post-treatment follow-up and 3–6 months follow-up. Finally, in terms of changes in cortical activity following treatment, while no group × time effects were observed on BOLD response during an n-back task, strengthened connectivity was observed in two of four networks analysed (the default mode network and the affective network) in the CR group relative to the control group based on a resting state analysis. Collectively these data provide evidence at behavioural, occupational and cortical levels for the efficacy of even low-support ‘e-health’-based CR interventions.
Low-support CR interventions
Following a meta-analysis of CR interventions for schizophrenia (Wykes et al. Reference Wykes, Huddy, Cellard, McGurk and Czobor2011), evidence of the benefits of CR is widespread, and also robust – with on average 0.45 s.d. improvements reported for patients receiving the intervention. The effects observed would appear to be relatively independent of the type of treatment provided – whether paper and pencil based, individual v. groups and (with few exceptions) the cognitive functions targeted. An important question, given this evidence of CR's efficacy, is how best to make CR available to patients in standard clinical care given the relatively limited resources available for psychological treatments in general, and in psychosis specifically. One approach to making psychological treatments more widely available has been via internet-based platforms – an approach described as ‘eHealth’, although the evidence base for these has been criticised as limited (Anthes, Reference Anthes2016). For patients with psychosis, the potential for adaptive computer-based training – training programmes that dynamically change in difficulty level according to patient ability – used in tandem with weekly therapist support rather than programmes that are purely 1:1, has received little attention to date. Given the cognitive and motivational challenges associated with psychosis, the feasibility of this approach is likely to be questioned by many clinicians. Here, however, in an unselected patient sample of chronic, middle-aged out-patients, more than half of the patients who began treatment were able to complete and in doing so, achieved a level of improvement comparable with that reported in the literature. The importance of these effects are likely to extend beyond WM-based training programmes to training of other cognitive deficits; the degree to which these, or indeed programmes targeting cognitive biases and metacognition, result in generalised improvement performance across multiple cognitive and social domains will be an important future research topic.
Neuropsychological effects
The specific targeting of WM in our programme was based on two factors – first, a desire to focus on an aspect of cognitive dysfunction whose underlying neural basis is relatively well established in schizophrenia (Lett et al. Reference Lett, Voineskos, Kennedy, Levine and Daskalakis2014; Constantinidis & Klingberg, Reference Constantinidis and Klingberg2016) and, secondly, the targeting of which may lead to more general benefits in cognition following even relatively little training. In a review of WM-based cognitive training in groups other than patients with psychosis, behavioural and neural studies have repeatedly associated WM ability with general cognitive ability, and WM training with general cognitive improvements, most likely by expanding the amount of information that can be represented and processed at the same time (Constantinidis & Klingberg, Reference Constantinidis and Klingberg2016). For schizophrenia, the decline in general cognitive ability reported in many patients makes targeting these deficits important. In the present study we observed that, in addition to improvements in untrained WM performance in the intervention group, improvements were also observed in episodic memory and in full scale IQ scores, the latter of which appeared to be driven by performance IQ (as measured by the WASI Matrix Reasoning subscale). That WM effects would generalise in terms of benefits to performance IQ rather than verbal IQ was not hypothesised, and may either reflect a null effect on verbal IQ or a difference in task sensitivity to change. However, a review of how WM training generalised to reasoning and intelligence tasks (Klingberg, Reference Klingberg2010) indicated that WM effects were almost always reported for non-verbal rather than verbal reasoning tasks. In schizophrenia, only one WM-based CR study to date reported level changes in ‘global cognition’, but only at trend level and did not parse this finding into verbal and performance IQ (Hargreaves et al. Reference Hargreaves, Dillon, Anderson-Schmidt, Corvin, Fitzmaurice, Castorina, Robertson and Donohoe2015). In the present study, our findings appear more consistent with those reported in non-psychotic samples.
Effects on social and occupational functioning
As noted in a recent review by Green (Reference Green2016), cognitive impairments have a significant impact on patients’ functional status, hence the need to target these impairments. An important corollary of this view is that improved cognitive performance should be associated with improved functional outcomes. In the present study, we measured change in function both on the basis of observer rated estimates of function (SOFAS), and patient task performance (ILS and UPSA-B). Despite almost identical scores at baseline, the CR group showed significant improvements in social and occupational function at both 2 weeks post-treatment and 3–6-month post-treatment outcomes, depending on the measure used. We interpret these changes as providing reliable evidence of improvements given that the changes observed were in the same direction across all measures assessed. Given that improved cognitive performance has been reported in schizophrenia without leading to changes in functional outcome (Fiszdon et al. Reference Fiszdon, Bryson, Wexler and Bell2004), it is interesting to note that Rispaud et al. recently found that it was WM related improvements that best predicted functional improvements in psychosis (Rispaud et al. Reference Rispaud, Rose and Kurtz2016).
These changes in function were observed in the absence of changes on the WHOQOL. Although we expected that improved cognition would be associated with benefits to quality of life, as for example Fizdon et al. recently reported (2016) (Fiszdon et al. Reference Fiszdon, Bryson, Wexler and Bell2004), no statistically significant changes in quality of life or self-esteem were observed. One interpretation of this is that these more psychological mediated benefits may take longer to become apparent. A high-intensity CR training study (Fisher et al. Reference Fisher, Holland, Subramaniam and Vinogradov2010; Subramaniam et al. Reference Subramaniam, Luks, Garrett, Chung, Fisher, Nagarajan and Vinogradov2014) targeting aspects of cognition including WM, observed significant gains in quality of life at 6-month follow-up but not before. Whether this longer follow-up period, or indeed the intensity or length of training were important factors leading to these benefits warrants further study.
Cortical effects of CR interventions
In recent reviews of cortical effects of CR training in schizophrenia (Ramsay & MacDonald, Reference Ramsay and MacDonald2015; Isaac & Januel, Reference Isaac and Januel2016), a majority showed training related reductions in cortical activity, with increased activations reported in a number of regions, including lateral and medial pre-frontal cortices. We observed no significant group × time effects on BOLD response during an n-back task, despite using a larger sample size than all but two previous studies. One reason for this may be the range in amount of time in training completed by patients in our CR group, which we argue is likely to approximate ‘real-world’ clinical settings more accurately.
Few studies to date have investigated the effects of CR on functional connectivity in psychosis. In the Penadés et al. (Reference Penadés, Pujol, Catalán, Massana, Rametti, García-Rizo, Bargalló, Gastó, Bernardo and Junqué2013) study, a functional connectivity analysis based on the default mode network and the central executive network suggested reduced connectivity following training in the CR group. To our knowledge, ours is the first study to report on functional connectivity during resting state in psychosis. Based on an analysis of functional connectivity changes in three networks that we have previously studied in schizophrenia (Mothersill et al. Reference Mothersill, Tangney, Morris, McCarthy, Frodl, Gill, Corvin and Donohoe2016), we found increased activation in two of these networks – namely the default mode network and the affective network. This finding is consistent with findings in two separate studies of enhanced functional connectivity in healthy controls following WM training (Jolles et al. Reference Jolles, van Buchem, Crone and Rombouts2013; Takeuchi et al. Reference Takeuchi, Taki, Nouchi, Hashizume, Sekiguchi, Kotozaki, Nakagawa, Miyauchi, Sassa and Kawashima2013). The direction of results differs from the Penadés et al. (Reference Penadés, Pujol, Catalán, Massana, Rametti, García-Rizo, Bargalló, Gastó, Bernardo and Junqué2013) study, however, although as already noted, their analysis was task rather than resting-state based.
A recent review of the cortical effects of WM suggested that increased functional connectivity between frontal and parietal cortices, and strengthened inter and intra-network connectivity, represent two of the five biological factors underlying training induced increases in capacity [others including increased neural firing rate and changes in striatal dopamine release and signalling (Constantinidis & Klingberg, Reference Constantinidis and Klingberg2016)]. For example, Jolles et al. (Reference Jolles, van Buchem, Crone and Rombouts2013) used fMRI to show that 6 weeks of WM training was associated with increased functional connectivity between frontal and parietal regions in healthy young adults (n = 15), and these changes were associated with increased accuracy on a WM task. Similarly, Thompson et al. (Reference Thompson, Waskom and Gabrieli2016) used fMRI to show that 20 days of WM training was associated with increases in frontoparietal functional connectivity in healthy young adults (n = 20), and that larger increases in connectivity were related to larger improvements in WM performance. These studies suggest that post-training increases in frontoparietal functional connectivity are beneficial and associated with increased WM capacity.
Our data support the theory that WM capacity depends on, and is strengthened by, both inter- and intra-network connectivity. For the default mode network, we observed strengthened inter-hemispheric connectivity between the right precuneous and the left inferior parietal lobule. In addition, we also observed strengthened connectivity between the left anterior and right middle cingulate. In terms of Constantinidis & Klingberg's review (Reference Constantinidis and Klingberg2016), rather than showing modulatory effects of pre-frontal cortex on parietal cortex, these findings suggest more intra-regional rather than inter-regional (e.g. top-down) strengthening of functional connectivity as the basis for increased capacity in patients with psychosis.
Study limitations
As already noted, when compared with cognitive training delivered either 1:1 or in groups, the dropout rate for this study was large, particularly for the intervention group. Similar dropout rates have recently been reported for other computer-based training initiatives (Fisher et al. Reference Fisher, Holland, Merzenich and Vinogradov2009). While we have argued that, in a chronic patient sample, the fact that half of all patients were able to complete the training is relatively good, further studies of remotely accessed (i.e. non-clinic-based) training programmes will benefit from consideration of the factors that could improve acceptability and adherence. For example, efforts to address issues of motivation and adherence may benefit from the ‘gamification’ of training platforms (i.e. using gaming environments to improve interest and appeal; see Savulich et al. Reference Savulich, Piercy, Fox, Suckling, Rowe, O’Brien and Sahakian2016; for examples). Of note, a majority of patients who dropped out of training did so in the first 1–2 weeks of training; on this basis either providing greater support at the start, or alternatively, more accurately identifying patients likely to experience difficulties may also offer a means of improving adherence.
Secondly, the amount of training undertaken by each patient varied widely, with many patients receiving significantly less than the 40 h of training often suggested as required to ensure durable benefits. For illustration, in addition to attending the weekly therapist support sessions, of the total sample recruited to the intervention arm of the study, the top 25% adherent group completed 32 or more hours or training, 50% of the sample completed 25 or more hours of training, and 75% of the sample complete 17 or more hours of training. Given the significant benefits in cognitive performance and function observed, this suggests that the benefits of cognitive training (at least for that focused on WM) requires less training to produce benefits than is typically advised. Of note, in a post hoc correlational analysis, no linear correlations between total time in training and cognitive benefits were observed on any outcome measure that showed significant change post-treatment. On the other hand, and offsetting these limitations in our view, is the low cost and potential increased accessibility of this training programme as part of routine clinical care. This is important because for those patients who can participate, the therapeutic benefits at a cognitive, social and cortical level, are comparable with other interventions with higher therapist staff costs (Wykes et al. Reference Wykes, Huddy, Cellard, McGurk and Czobor2011). However, the extent to which the increased training variability associated with remote training may affect generalisability of cognitive improvements remains to be investigated.
Finally, the number of patients in each arm of the study who underwent MRI was relatively large for CR studies, but nonetheless was likely to have been underpowered to identify subtle training related differences. While recent meta-analyses in this area highlight the cortical benefits of this training, the need for better powered imaging analysis remains. So also does the need to specify the relationship between the improved cognitive and social function observed and the changes in cortical function observed. Based on the present study, a reasonable hypothesis to pursue in future research is that increased cortical activity may be causally related to both improved cognitive performance and improved social function. Whether this is the case either directly or via mediation (e.g. whereby cognitive improvements mediate the effects of cortical changes on social functioning) remains to be investigated in a follow-on study.
Conclusion
CR training is reliably associated with improved cognitive performance on non-trained tasks, and with changes in cortical networks, particularly in terms of enhanced inter-hemispheric connectivity within prefrontal and parietal cortex. These benefits are, furthermore, associated with benefits to social and occupational functioning, aspects of psychosis related disability not addressed by pharmacological treatments. Given the limited resources available within many health services for delivering CR training, the evidence that even limited amounts of training, delivered remotely and with low support, can lead to such benefits is important. In the context of current knowledge about training, it provides further evidence that suggests CR can be made available in a manner that is relatively low cost and produce benefits that are durable.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291717001982.
Acknowledgements
We thank all patients and staff who participated in the collection of patient data. Recruitment of the patient sample was supported by a Health Research Board (Ireland) grant to GD.
Declaration of Interest
All authors confirm that they have no conflicts of interest in relation to this manuscript.