Introduction
The risk for developing major depressive disorder (MDD) is increased when familial risk (i.e. having a first-degree relative with a psychiatric disorder) or childhood maltreatment (CM) are present in a person (Nanni, Uher, & Danese, Reference Nanni, Uher and Danese2012; Rasic, Hajek, Alda, & Uher, Reference Rasic, Hajek, Alda and Uher2014). Yet, both risk factors only explain a small proportion of the variance in actual symptom presentation (Colodro-Conde et al., Reference Colodro-Conde, Couvy-Duchesne, Zhu, Coventry, Byrne, Gordon and Martin2018; Lieb, Isensee, Höfler, Pfister, & Wittchen, Reference Lieb, Isensee, Höfler, Pfister and Wittchen2003; Nanni et al., Reference Nanni, Uher and Danese2012). Besides risk factors that increase the risk for MDD, other factors must be at play that simultaneously mitigate these risks.
Familial risk increases the risk of developing the disorder from which the parent suffered (risk ratio, RR = 3.59), but also the risk for other psychiatric disorders (RR = 1.92) (Rasic et al., Reference Rasic, Hajek, Alda and Uher2014). It is regarded as a proxy for genetic liability, but could also lead to a more stressful environment (Flory, Yehuda, Passarelli, & Siever, Reference Flory, Yehuda, Passarelli and Siever2012). Healthy relatives with familial risk for MDD show similar gray matter volume (GMV) alterations as MDD patients, specifically reductions in the insula and orbitofrontal cortex (OFC) (Opel et al., Reference Opel, Zwanzger, Redlich, Grotegerd, Dohm, Arolt and Dannlowski2016).
The environmental risk factor CM is associated with increased risk for developing depression (odds ratio, OR = 2.66–3.73), and within patients, CM is associated with recurrent or persistent depressive episodes (OR = 2.27) (Nanni et al., Reference Nanni, Uher and Danese2012; Nelson, Klumparendt, Doebler, & Ehring, Reference Nelson, Klumparendt, Doebler and Ehring2017; Teicher & Samson, Reference Teicher and Samson2013). It is associated with increased social problems, negative cognitive bias, increased limbic responsiveness to negative stimuli, alterations in brain connectivity patterns, and persistent pro-inflammatory states of the immune system, among others (Baumeister, Akhtar, Ciufolini, Pariante, & Mondelli, Reference Baumeister, Akhtar, Ciufolini, Pariante and Mondelli2016; Dannlowski et al., Reference Dannlowski, Kugel, Huber, Stuhrmann, Redlich, Grotegerd and Suslow2013; Günther, Dannlowski, Kersting, & Suslow, Reference Günther, Dannlowski, Kersting and Suslow2015; Meinert et al., Reference Meinert, Repple, Nenadic, Krug, Jansen, Grotegerd and Dannlowski2019; Opel et al., Reference Opel, Redlich, Dohm, Zaremba, Goltermann, Repple and Dannlowski2019; Ouellet-Morin et al., Reference Ouellet-Morin, Odgers, Danese, Bowes, Shakoor, Papadopoulos and Arseneault2011; Redlich et al., Reference Redlich, Opel, Bürger, Dohm, Grotegerd, Förster and Dannlowski2018). In healthy participants with CM, GMV changes were reported in the hippocampus, corpus callosum, anterior cingulate, OFC, and dorsolateral prefrontal cortex (DLPFC) (Dannlowski et al., Reference Dannlowski, Kugel, Grotegerd, Redlich, Opel, Dohm and Baune2016, Reference Dannlowski, Stuhrmann, Beutelmann, Zwanzger, Lenzen, Grotegerd and Kugel2012; Teicher, Samson, Anderson, & Ohashi, Reference Teicher, Samson, Anderson and Ohashi2016).
Although there is a great focus in research on CM, few studies have investigated the combined effects of both CM and familial risk. The literature suggests that the presence of both risk factors in the same individual is associated with even worse mental health outcomes: in adolescents, this twofold risk was found to increase risk for several psychiatric disorders (MDD, autism spectrum disorders, conduct disorder, and generalized anxiety disorder) as well as to increase risk to attempt suicide, and increase risk of suicidal behavior up to OR = 4.43 (Greger, Myhre, Lydersen, & Jozefiak, Reference Greger, Myhre, Lydersen and Jozefiak2015; Zelazny et al., Reference Zelazny, Melhem, Porta, Biernesser, Keilp, Mann and Brent2019). In a study by Flory et al. (Reference Flory, Yehuda, Passarelli and Siever2012), such participants had an almost sixfold risk to develop post-traumatic stress disorder (PTSD) (OR = 5.89). A decreased volume in the bilateral hippocampal heads, in the DLPFC, medial prefrontal cortex (MPFC) and anterior cingulate cortex was reported in healthy participants with familial risk for MDD and history of emotional abuse (a subscale of the childhood trauma questionnaire) (Carballedo et al., Reference Carballedo, Lisiecka, Fagan, Saleh, Ferguson, Connolly and Frodl2012).
The risk factors CM and familial risk entail grave, adverse effects for the individual and are associated with morphometric changes already present in healthy individuals. However, the data clearly show that even the presence of both risks does not inevitably lead to the development of MDD. Clearly, such healthy high-risk individuals must be equipped with resources, positive coping skills, or traits which enable them to maintain mental health. Although many studies focus on the negative impact of risk factors, few studies investigate how some individuals at high risk still manage to maintain mental health, i.e. why they are resilient.
Resilience is a protective mechanism which is defined as the dynamic process to successfully adapt and cope with trauma, adversity, and negative stressors (Newman, Reference Newman2005; Windle, Reference Windle2011). Resilient individuals cope better with stress, report more positive emotions and are less likely to report depressive symptoms (Klasen et al., Reference Klasen, Otto, Kriston, Patalay, Schlack and Ravens-Sieberer2015; Ong, Bergeman, Bisconti, & Wallace, Reference Ong, Bergeman, Bisconti and Wallace2006). The concept of resilience encompasses both individual traits and skills, but also favorable environment, such as social support, intelligence quotient (IQ), and socioeconomic status (Ettner, Reference Ettner1996; Ozbay, Fitterling, Charney, & Southwick, Reference Ozbay, Fitterling, Charney and Southwick2008). The operationalization of resilience is a topic of fierce discussion, and operationalization in different studies varies greatly (Chmitorz et al., Reference Chmitorz, Kunzler, Helmreich, Tüscher, Kalisch, Kubiak and Lieb2018; Kalisch, Müller, & Tüscher, Reference Kalisch, Müller and Tüscher2015; Luthar, Cicchetti, & Becker, Reference Luthar, Cicchetti and Becker2000). Some studies identify resilient individuals as those who do not develop PTSD after a traumatic event, which can only represent resilience to extreme events (Yehuda, Flory, Southwick, & Charney, Reference Yehuda, Flory, Southwick and Charney2006). Some rely on self-report questionnaires to assess resilience, which is termed trait resilience (Kahl, Wagner, de la Cruz, Köhler, & Schultz, Reference Kahl, Wagner, de la Cruz, Köhler and Schultz2018; Kalisch et al., Reference Kalisch, Cramer, Binder, Fritz, Leertouwer, Lunansky and van Harmelen2019; Pangallo, Zibarras, Lewis, & Flaxman, Reference Pangallo, Zibarras, Lewis and Flaxman2015). These questionnaires measure skills such as cognitive reframing, ability to bounce back, acceptance, and personal competence, which have all been identified as skills relevant to resilience (Smith et al., Reference Smith, Dalen, Wiggins, Tooley, Christopher and Bernard2008; Wagnild & Young, Reference Wagnild and Young1993). Direct stimulation of the left DLPFC (lDLPFC) and OFC using trans-cranial direct current stimulation (tDCS), increased levels of trait resilience as measured with a resilience questionnaire (Salehinejad, Nejati, & Derakhshan, Reference Salehinejad, Nejati and Derakhshan2017). Using questionnaires to assess resilience has shortcomings: first, self-reported resilience does not necessarily portray objective resilience, and second, without assessing adversity, questionnaires cannot represent the entire, process-like concept of resilience.
Other studies identify healthy, but at-risk individuals (e.g. with CM) as resilient. Resilience to CM has been associated with changes in the MPFC, and functional alterations in limbic areas such as hippocampus and amygdala (Ioannidis, Askelund, Kievit, & Van Harmelen, Reference Ioannidis, Askelund, Kievit and Van Harmelen2020; Moreno-López et al., Reference Moreno-López, Ioannidis, Askelund, Smith, Schueler and van Harmelen2020). High-risk but healthy adolescents were shown to have higher GMV in the right middle and superior frontal gyri (Burt et al., Reference Burt, Whelan, Conrod, Banaschewski, Barker, Bokde and Garavan2016). In a longitudinal investigation, it was found that those individuals who remained healthy despite high-risk were better able to access prefrontal regions for emotion regulation (Rodman, Jenness, Weissman, Pine, & McLaughlin, Reference Rodman, Jenness, Weissman, Pine and McLaughlin2019).
Although results are mixed, it seems that the broader construct of resilience is associated with alterations in (pre)frontal regions that aid in planning, appraisal, executive functioning, and emotion regulation. Neuroimaging studies that identify healthy high-risk individuals as resilient have methodological shortcomings. In these, correlates of resilience are identified by either comparing healthy low-risk to healthy high-risk participants, or by comparing healthy high-risk to depressed high-risk participants. Although it might be possible to speculate about ‘neural resilience correlates’ using these comparisons, the former approach can de facto only identify the effects of risk status, while the latter can only identify the effects of diagnosis status (Amico et al., Reference Amico, Meisenzahl, Koutsouleris, Reiser, Möller and Frodl2011).
Indeed, the interactive effect of risk and protective factors was reported in two studies at a phenotypical level, demonstrating risk and resilience contributed toward the likelihood of manifestation of MDD symptoms in an opposing, yet interactive manner (Navrady, Adams, Chan, Ritchie, & McIntosh, Reference Navrady, Adams, Chan, Ritchie and McIntosh2017; Wingo et al., Reference Wingo, Wrenn, Pelletier, Gutman, Bradley and Ressler2010). These findings highlight that it is not the mere absence of risk factors that help maintain mental health but also the presence of resilience factors, and that the relationship between risk and resilience is complex and interactive.
In summary, (1) there is a lack of studies that investigate the subgroup of healthy high-risk individuals with both CM and familial risk, (2) other studies investigating risk factors have methodological shortcomings in the operationalization of resilience, and (3) only the interaction of risk × diagnosis can give information about resilience-specific morphometric alterations.
In the current study, high risk is operationalized as the presence of the two risk factors CM and familial risk. Low risk is operationalized as the absence of both CM and familial risk. Resilience is operationalized as maintaining mental health despite the presence of the two risk factors CM and familial risk. In our study, we aim to examine brain morphometric correlates of resilience of healthy, high-risk participants, compared to healthy low-risk participants, depressed high-risk participants, and depressed low-risk participants. We employ a 2 × 2 group design: risk (high risk v. low risk) × diagnosis: (healthy v. depressed), investigating the interaction effect. Based on the literature, we expect to find higher volume in frontal regions which might counteract the adverse effects of risk and aid in the maintenance of mental health.
Methods and materials
Sample
For the current study, we selected a sample of N = 804 subjects of the FOR2107 cohort (Marburg-Münster Affective Disorder Cohort Study) study (http://www.for2107.de). FOR2107 is an ongoing longitudinal, bicentric cohort study investigating the neurobiology of affective [major depression and bipolar disorder (BP)] and psychotic disorders [schizophrenia (SZ) and schizoaffective disorder]. Participants are deeply phenotyped using questionnaires, neuropsychological testing, neuroimaging (functional and structural), and collection of biomaterial (for a detailed description see Kircher et al., Reference Kircher, Wöhr, Nenadic, Schwarting, Schratt, Alferink and Dannlowski2018). Data are collected at baseline, and 2 and 5 years afterward. Data for this study were collected between 2014 and 2018, with a median of data collected in 2016. Healthy participants, and patients (both acute and remitted) were recruited via newspaper advertisements; patients were additionally recruited via local in- and outpatient services in Marburg and Münster, Germany. Written informed consent was obtained from all subjects before participation, and participants received a financial compensation afterward. Participants with a history of substance dependence, neurological disorders, severe medical disorders, head trauma, or IQ <80 were excluded. We included participants aged 18–65. Healthy participants with no history of mental illness were included. The study was approved by the ethics committees of the respective recruitment sites in accordance with the Declaration of Helsinki. The German version of the structured clinical interview for DSM-IV-TR (SCID-I) (Wittchen, Gruschwitz, Wunderlich, & Zaudig, Reference Wittchen, Gruschwitz, Wunderlich and Zaudig1997) was used to diagnose participants by trained raters.
We employed a 2 × 2 (diagnosis × risk) design, distinguishing healthy participants (HC) and depressed patients (MDD) (factor diagnosis) at either low risk (R−) or high risk for MDD (R+) (factor risk). R− reported neither familial risk nor CM, while R+ reported both familial risk and CM. The descriptive data of the sample can be found in Table 1.
Table 1. Descriptive sample statistics

*The significant of bold values indicated p values < 0.05.
The mean values are reported, with the standard deviation in parentheses, unless otherwise specified. Bonferroni post-hoc tests were used to compare the groups.
HCR−, healthy control and low risk; HCR+, healthy control and high risk; MDDR−, depressive and low risk; MDDR+, depressive and high risk; HAM-D, Hamilton Depression Rating Scale, BDI, Beck Depression Inventory.
a HC groups differ significantly from MDD groups, MDD groups differ significantly from each other: HCR− < MDDR−; HCR− < MDDR+; HCR+ < MDDR−; HCR+ < MDDR+; MDDR− < MDDR+.
Assessment of familial risk
Familial risk was defined as having a first-degree relative with MDD, BP, or SZ, and assessed as part of a questionnaire battery, asking if the participant had a parent, sibling, or child who had ever been treated for one of these disorders. In the R− group, only participants not reporting any psychiatric problems in any first-degree relatives were included.
Assessment of CM
The German version of the Childhood Trauma Questionnaire (CTQ) was used to assess CM retrospectively (Wingenfeld et al., Reference Wingenfeld, Spitzer, Mensebach, Grabe, Hill, Gast and Driessen2010). This self-report questionnaire consists of five subscales: emotional abuse, physical abuse, sexual abuse, emotional neglect, physical neglect; with five items per scale rated on a 5-point Likert scale. A higher score indicates a higher degree of maltreatment. Individuals were classified as having experienced CM when the respective cut-off score according to Walker et al. (Reference Walker, Gelfand, Katon, Koss, Von Korff, Bernstein and Russo1999) was met in at least one of the five scales.
Magnetic resonance imaging (MRI) data acquisition
At both sites, high-resolution T1 images were acquired with a 3T whole body MRI scanner (Marburg: 12-channel head matrix Rx-coil, Tim Trio, Siemens, Erlangen, Germany; Münster: 20-channel head matrix Rx-coil, Prisma, Siemens, Erlangen, Germany). A 3D fast gradient echo sequence (MPRAGE) was used with the following configurations: Marburg: field of view (FOV) = 256 mm, 176 slices, repetition time (TR) = 1900 ms, echo time (TE) = 2.26 ms, inversion time (TI) = 900 ms, slice thickness = 1 mm, voxel size = 1 × 1 × 1 mm, flip angle = 9° and Münster: FOV = 256 mm, 192 slices, TR = 2130 ms, TE = 2.28 ms, TI = 900 ms, slice thickness = 1 mm, voxel size = 1 × 1 × 1 mm, flip angle 8°. Image quality was assessed using the quality assurance protocols of the CAT12 toolbox.
The CAT12 toolbox (build 1184, Gaser, Structural Brain Mapping group, Jena University Hospital, Jena, Germany) implemented in SPM12 (v7771, Statistical Parametric Mapping, Institute of Neurology, London, UK), running under MATLAB (version v2017a, The MathWorks, USA) was used for pre-processing of scans using default parameter settings. Images were segmented into gray matter, white matter, and cerebrospinal fluid and spatially normalized using the DARTEL algorithm. All images passed individual quality control, checking for artifacts and image quality. Data were smoothed with an 8 mm full-width half-maximum Gaussian kernel, using an absolute threshold of 0.1.
Statistical analyses
Data were analyzed using SPSS25 and SPM12. We conducted an analysis of covariance (ANCOVA) interaction analysis using a 2 × 2 design in SPM, including the covariates age, sex, total intracranial volume, as well as body coil and site, in accordance with our MRI quality assurance protocol (Vogelbacher et al., Reference Vogelbacher, Möbius, Sommer, Schuster, Dannlowski, Kircher and Bopp2018). We examined four bilateral ROIs and applied small volume correction using family-wise error (FWE) correction. ROIs were defined using the Neuromorphometrics atlas implemented in SPM12.
To examine the interaction effect of significant ROIs, we extracted eigenvariate values, which are an approximation of mean volume inside the cluster and compared them in SPSS using ANCOVA (correcting for all covariates).
ROI selection
Drawing on the literature and our hypotheses, we selected four bilateral ROIs that had previously been implicated in both resilience research and the investigation of healthy high-risk participants (CM and/or familial risk) for the interaction analysis. Overlapping areas in both risk and resilience research are (pre)frontal areas. We therefore selected four bilateral ROIs using the Neuromorphometrics atlas: middle frontal gyrus (MFG) (Burt et al., Reference Burt, Whelan, Conrod, Banaschewski, Barker, Bokde and Garavan2016; Carballedo et al., Reference Carballedo, Lisiecka, Fagan, Saleh, Ferguson, Connolly and Frodl2012; Salehinejad et al., Reference Salehinejad, Nejati and Derakhshan2017), superior frontal gyrus (Burt et al., Reference Burt, Whelan, Conrod, Banaschewski, Barker, Bokde and Garavan2016), inferior frontal gyrus (orbital part) (Opel et al., Reference Opel, Zwanzger, Redlich, Grotegerd, Dohm, Arolt and Dannlowski2016), and frontal pole (Carballedo et al., Reference Carballedo, Lisiecka, Fagan, Saleh, Ferguson, Connolly and Frodl2012; Moreno-López et al., Reference Moreno-López, Ioannidis, Askelund, Smith, Schueler and van Harmelen2020; Teicher et al., Reference Teicher, Samson, Anderson and Ohashi2016).
Results
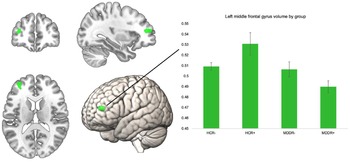
To investigate the interactive effect of diagnosis and risk on GMV, we performed an interaction analysis using the four bilateral ROIs. The interaction analysis did not reveal significant findings for the bilateral superior frontal gyri, the frontal poles, pars orbitalis of the inferior frontal gyrus, and the right MFG, p > 0.05. There was a significant effect for the left MFG ROI at peak level (k = 199, x/y/z = −34/48/12, T = 4.06, p = 0.047, FWE corrected). To examine this interaction effect by group, we extracted eigenvariate values of this cluster and compared them in SPSS using an ANCOVA (correcting for all covariates) (Fig. 1).
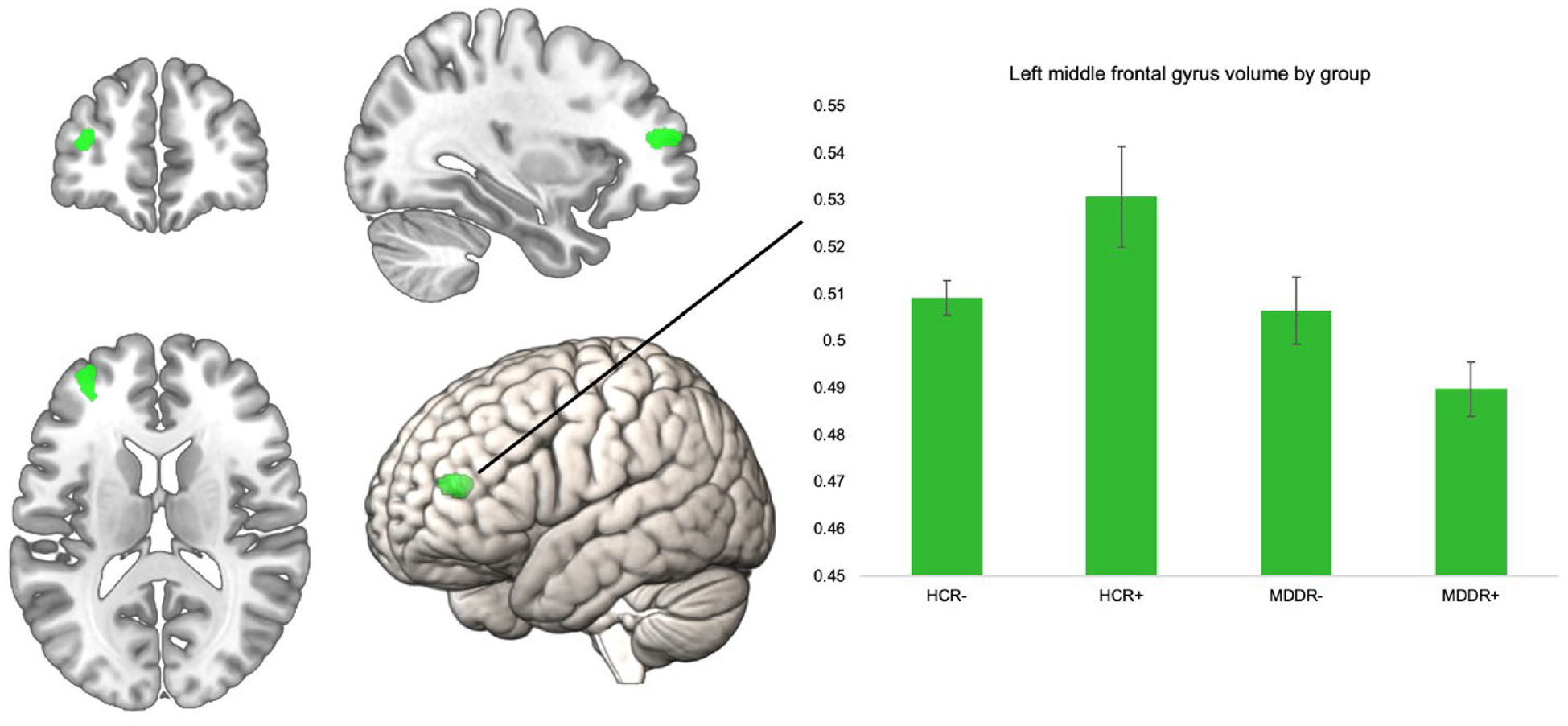
Fig. 1. Significant left MFG ROI for interaction of risk × diagnosis, volume in the left MFG by group. Error bars indicate 1 standard error of the mean.
The groups differed significantly in their left MFG volume F (3,796) = 10.32, p < 0.001, η 2 = 0.037. We compared HCR+ to the other three groups using Bonferroni post-hoc comparisons. HCR+ had significantly higher volumes compared to all other groups. HCR+ v. HCR−; HCR+ v. MDDR−, and HCR+ v. MDDR+: all p < 0.001. The ROI and the mean volumes per group are shown below. Including body mass index or years of education as an additional covariate in the model did not alter the results, nor did excluding obvious GMV outliers (see online Supplementary Table 2). To further investigate robustness of our finding, we analyzed this interaction effect in only women (online Supplementary Table 2), and in an additional sample of healthy controls with intermediate risk (online Supplementary Table 3). The supplement also lists the results of the whole-brain analysis (online Supplementary Table 4).
Discussion
Our results reveal higher volume in the left MFG, part of the DLFPC, in healthy participants with two risk factors (CM and familial risk) for MDD, compared to depressed patients irrespective of risk status, and compared to healthy low-risk individuals. To our knowledge, this is the first study investigating GMV in healthy high-risk (both CM and familial risk) in a 2 × 2 design. The DLFPC is associated with cognitive flexibility, reappraisal, and impulse control (Steinbeis, Bernhardt, & Singer, Reference Steinbeis, Bernhardt and Singer2012). These skills are known to contribute to mental health and are impaired in MDD. Increased volume in this region could mitigate risk effects in already at-risk individuals. Skills such as reframing and cognitive flexibility have already been identified as parts of resilience (Iacoviello & Charney, Reference Iacoviello and Charney2014). The DLPFC might not only be implicated in cognitive processes, but also in emotional ones: administration of repetitive transcranial magnetic stimulation to the left DLFPC in MDD patients increased empathetic happiness and decreased anhedonia (Light, Bieliauskas, & Taylor, Reference Light, Bieliauskas and Taylor2019). Using tDCS of the lDLPFC combined with gaze-contingent training was shown to improve attention regulation in low-resilient individuals (Sanchez-Lopez et al., Reference Sanchez-Lopez, De Raedt, Puttevils, Koster, Baeken and Vanderhasselt2020). DLPFC volume was furthermore shown to be a mediator between socioeconomic status and executive functioning (Shaked et al., Reference Shaked, Katzel, Seliger, Gullapalli, Davatzikos, Erus and Waldstein2018). This also highlights the importance of environmental factors important in the maintenance of mental health (Ettner, Reference Ettner1996; Ozbay et al., Reference Ozbay, Fitterling, Charney and Southwick2008). Our results point to the lDLPFC as a neural correlate of resilience in high-risk individuals. Higher volume in the DLPFC might aid these at-risk individuals both on a cognitive- and on an emotional level in maintaining mental health.
It is important to note that healthy, high-risk individuals with both CM and familial risk constitute a unique subgroup of healthy participants. Drawing from a sample of n = 1500 healthy individuals, only n = 67 fit the criteria for HCR+, while n = 437 healthy individuals reported no such risk factor. On the other hand, risk distribution in MDD was remarkably different; here, n = 199 depressive participants fit the same risk criteria (χ2(1, N = 804) = 238.97, p < 0.001). This shows that our HCR+ group constitutes a rare, highly resilient subgroup that can only be investigated in a large sample of participants. Our results point to the lDLFPC as a protective morphometric correlate of resilience that aids participants with both CM and familial risk in the maintenance of mental health.
Limitations
We limited detection to previously selected ROIs. These ROIs were based on solid previous research and enabled us to purposefully identify regions associated with resilience. Our groups cannot represent the heterogeneity of MDD patients or risk groups, as we excluded participants with only CM or familial risk present. However, they do provide valuable insight into the understanding of high- and low-risk groups. Environmental risk was assessed using the CTQ questionnaire, which – as a self-report instrument – has strengths and limitations (Baldwin, Reuben, Newbury, & Danese, Reference Baldwin, Reuben, Newbury and Danese2019; Goltermann, Opel, & Dannlowski, Reference Goltermann, Opel and Dannlowski2019; MacDonald et al., Reference MacDonald, Thomas, Sciolla, Schneider, Pappas, Bleijenberg and Wingenfeld2016). Participants were identified as having CM when they exceeded at least one of the five subscales of the CTQ. This approach can be problematic, and a continuous assessment of maltreatment (using the sum score) would be valuable, however, this was not feasible using this model. Our groups can still separate between high- and low-risk, as low-risk groups did not have either familial risk, nor exceed one scale of the CTQ. We included both remitted and acute MDD patients, which might have made MDD groups more heterogenous. Familial risk was defined as having a first-degree relative with MDD, BP, or SZ. Distinct morphometric changes have been described for the relatives of MDD and SZ patients, but linkage and recent genome-wide association studies have emphasized the substantial genetic overlap among BP, SZ, and MDD (Anttila et al., Reference Anttila, Bulik-Sullivan, Finucane, Walters, Bras, Duncan and Neale2018; McDonald et al., Reference McDonald, Bullmore, Sham, Chitnis, Wickham, Bramon and Murray2004; Smoller et al., Reference Smoller, Andreassen, Edenberg, Faraone, Glatt and Kendler2019). We operationalized resilience as the outcome of mental health, despite the presence of CM and familial risk. Trait questionnaires would be able to assess self-reported resilience skills such as reframing and optimism. However, mental health despite high risk is a much more objective and direct measure of resilience than subjective, self-reported resilience.
Future directions
Our study identifies morphometric correlates of resilience in a highly resilient subgroup of individuals at high risk (both CM and familial risk) to develop MDD. This group should be examined in more detail in future research. In our study, HCR+ did not show uniquely higher scores in other relevant measures such as IQ, income, education, or trait resilience (see online Supplementary Table 1). Studies investigating the effects of MDD and other psychiatric disorders should take into account the effects of risk and resilience, as they both contribute in a unique way to either increase or decrease the risk for these disorders. The concrete cognitive and behavioral correlates of higher volume in the lDLPFC, which might be improved emotional attentional control and cognitive flexibility, among others, should be investigated in future studies.
Bolstering resilience by teaching such techniques should be integrated as a central goal in both prevention and psychotherapy. Resilience seems to be the default response to adversity, rather than the exception. Considering the grave impact of risk factors, this adaptive coping equals an ‘ordinary magic’, and resilience factors both on a morphometric, and a behavioral level deserve more attention in future research (Masten, Reference Masten2001).
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291721001094.
Acknowledgements
This study is part of the German multicenter consortium ‘Neurobiology of Affective Disorders. A translational perspective on brain structure and function’, funded by the German Research Foundation (Deutsche Forschungsgemeinschaft DFG; Forschungsgruppe/Research Unit FOR2107). We are deeply indebted to all study participants and staff. A list of acknowledgments can be found at: for2107.de/acknowledgements.
Conflict of interest
Biomedical financial interests or potential conflicts of interest: Tilo Kircher received unrestricted educational grants from Servier, Janssen, Recordati, Aristo, Otsuka, and neuraxpharm. All other authors declare no conflict of interest.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.