Introduction
Major depressive disorder (MDD) is one of the most common mental disorders and a leading cause of years lived with disability (James et al., Reference James, Abate, Abate, Abay, Abbafati, Abbasi and Murray2018). A core symptom of MDD, according to both DSM-V and ICD-10 criteria, is anhedonia, an array of deficits impacting various hedonic functions such as desire, motivation and pleasure (Rizvi, Pizzagalli, Sproule, & Kennedy, Reference Rizvi, Pizzagalli, Sproule and Kennedy2016). Patients suffering from anhedonia show overall poorer treatment response (Spijker, Bijl, Graaf, & Nolen, Reference Spijker, Bijl, Graaf and Nolen2001; Vrieze et al., Reference Vrieze, Demyttenaere, Bruffaerts, Hermans, Pizzagalli, Sienaert and Claes2014), possibly because preliminary evidence suggests that established pharmacotherapies, particularly selective serotonin reuptake inhibitors, are not well suited to treat motivational and reward-related dysfunctions in depression (Dunlop & Nemeroff, Reference Dunlop and Nemeroff2007; McCabe, Mishor, Cowen, & Harmer, Reference McCabe, Mishor, Cowen and Harmer2010). On a neurobiological level, anhedonia has been associated with the reward network (for an overview, see Höflich, Michenthaler, Kasper, & Lanzenberger, Reference Höflich, Michenthaler, Kasper and Lanzenberger2019). Meta-analytical evidence from neuroimaging studies shows that patients with MDD exhibit reduced responses to monetary incentives and happy faces in various reward network nodes, such as the nucleus accumbens, caudate, putamen, insula and orbitofrontal cortex (Keren et al., Reference Keren, O'Callaghan, Vidal-Ribas, Buzzell, Brotman, Leibenluft and Stringaris2018; Ng, Alloy, & Smith, Reference Ng, Alloy and Smith2019; Zhang, Chang, Guo, Zhang, & Wang, Reference Zhang, Chang, Guo, Zhang and Wang2013). Moreover, higher reward sensitivity is associated with better outcome after psychotherapeutic interventions (Papalini et al., Reference Papalini, Lange, Bakker, Michielse, Marcelis, Wichers and Schruers2019).
Social interactions are considered natural rewards (Insel, Reference Insel2003) and activate the reward network in healthy participants (Alkire, Levitas, Warnell, & Redcay, Reference Alkire, Levitas, Warnell and Redcay2018; Izuma, Saito, & Sadato, Reference Izuma, Saito and Sadato2008; Kawamichi et al., Reference Kawamichi, Sugawara, Hamano, Makita, Kochiyama and Sadato2016; Redcay et al., Reference Redcay, Dodell-Feder, Pearrow, Mavros, Kleiner, Gabrieli and Saxe2010). Even though MDD patients often suffer from impairments in social functioning (for an overview, see Kupferberg, Bicks, & Hasler, Reference Kupferberg, Bicks and Hasler2016), few studies have probed the processing of social reward in MDD (Hsu et al., Reference Hsu, Sanford, Meyers, Love, Hazlett, Walker and Zubieta2015; Olino, Silk, Osterritter, & Forbes, Reference Olino, Silk, Osterritter and Forbes2015). For instance, social touch can be inherently rewarding and is an integral part of nonverbal social communication and bonding (Hertenstein, Verkamp, Kerestes, & Holmes, Reference Hertenstein, Verkamp, Kerestes and Holmes2006; Morrison, Löken, & Olausson, Reference Morrison, Löken and Olausson2010), but it is still elusive whether MDD also modulates the processing of rewarding interpersonal, tactile stimulation.
Social distancing measures in the era of COVID-19 have vividly demonstrated the importance of interpersonal touch and the consequences of its absence. Social touch deprivation during the pandemic has been linked to increased anxiety and loneliness and resulted in a craving for interpersonal touch (von Mohr, Kirsch, & Fotopoulou, Reference von Mohr, Kirsch and Fotopoulou2021). The processing of touch is mediated by different pathways in the nervous system. Myelinated Aβ-fibers enable rapid central processing and convey discriminative information, allowing for prompt responses to a stimulus. These fibers are preferentially activated by fast tactile stimulation, whereas unmyelinated C-tactile (CT) afferents respond to slow, caressing stimulation that corresponds to rewarding and affective properties of touch with increased firing frequency (McGlone, Wessberg, & Olausson, Reference McGlone, Wessberg and Olausson2014). Functional magnetic resonance imaging (fMRI) studies indicate a possible association between interpersonal touch and the reward circuit. Being touched by another person, but not self-produced touch, increases neural activation in the caudate nucleus (Boehme, Hauser, Gerling, Heilig, & Olausson, Reference Boehme, Hauser, Gerling, Heilig and Olausson2019). Intranasal oxytocin, a neuropeptide crucially involved in social bonding, increases nucleus accumbens activity when participants believe they are being touched by their romantic partner (Kreuder et al., Reference Kreuder, Scheele, Wassermann, Wollseifer, Stoffel-Wagner, Lee and Hurlemann2017). Similarly, increased pleasantness ratings and striatal activity have been observed when heterosexual male participants believe social touch is being delivered by a female as opposed to a male experimenter (Scheele et al., Reference Scheele, Kendrick, Khouri, Kretzer, Schläpfer, Stoffel-Wagner and Hurlemann2014; Zimmermann et al., Reference Zimmermann, Kendrick, Scheele, Dau, Banger, Maier and Becker2019). Striatal response to affective touch seems to increase with age (May, Stewart, Paulus, & Tapert, Reference May, Stewart, Paulus and Tapert2014).
Besides the assumed involvement of the reward network, other pathological features of MDD might also affect the processing of social touch. Cognitive biases, such as the negativity bias, are common in MDD and are associated with blunted responses to positive stimuli in striatal regions, the amygdala and the thalamus (Diener et al., Reference Diener, Kuehner, Brusniak, Ubl, Wessa and Flor2012; Groenewold, Opmeer, de Jonge, Aleman, & Costafreda, Reference Groenewold, Opmeer, de Jonge, Aleman and Costafreda2013). While interoceptive dysfunctions traditionally have not been regarded as a core symptom of depression, increasing evidence points toward substantial impairments in the perception of bodily signals (Harshaw, Reference Harshaw2015; Paulus & Stein, Reference Paulus and Stein2010) and related neural representations in the insular cortex (Avery et al., Reference Avery, Drevets, Moseman, Bodurka, Barcalow and Simmons2014) in MDD patients. Recently, the perception of affective touch has been discussed as an interoceptive signal (Crucianelli & Ehrsson, Reference Crucianelli and Ehrsson2023) and might therefore be sensitive to pathologically altered interoception in MDD.
The rationale of the present study was to probe whether MDD is associated with altered processing of social touch. We therefore examined patients with MDD before and after a multi-week course of antidepressant treatment and compared them to healthy controls who were examined over the same period. We employed a social touch fMRI paradigm, during which participants rated the comfort of slow and fast touch. Additionally, we assessed depressive symptom severity over the course of the study in MDD patients. We expected MDD patients to perceive social touch as less comfortable and to display decreased neural responses to social touch compared to healthy controls, particularly in regions associated with blunted neural response to reward in MDD patients: the nucleus accumbens, caudate nucleus, putamen and insula (Hsu et al., Reference Hsu, Sanford, Meyers, Love, Hazlett, Walker and Zubieta2015; Keren et al., Reference Keren, O'Callaghan, Vidal-Ribas, Buzzell, Brotman, Leibenluft and Stringaris2018; Zhang et al., Reference Zhang, Chang, Guo, Zhang and Wang2013). We further hypothesized that these MDD-related alterations would decrease after treatment. Since anhedonia is associated with worse treatment outcome, we expected that non-responders to antidepressant therapy would report lower comfort ratings and exhibit lower neural responses to social touch compared to responders. We assumed that these effects would be particularly pronounced in response to slow as opposed to fast touch.
Materials and methods
Participants and study design
Between June 2016 and April 2018, 53 patients with MDD (27 female, age 41.58 ± 13.09 years) and 41 healthy controls (22 female, age 40.61 ± 13.22 years) participated in this study (Table 1). To participate in this registered study (https://clinicaltrials.gov/show/NCT04081519), all patients had to meet DSM-IV criteria for unipolar MDD as diagnosed by an experienced psychiatrist and verified by the Mini-International Neuropsychiatric Interview (MINI; Sheehan et al., Reference Sheehan, Lecrubier, Sheehan, Amorim, Janavs, Weiller and Dunbar1998), and were in-patients at the Department of Psychiatry, University Hospital Bonn, Germany. Exclusion criteria for all participants were suicidal ideation, psychotic symptoms, bipolar depression, substance abuse, eating disorders, post-traumatic stress disorder, personality disorders, neurological disorders and MRI contraindications. For healthy controls, additional exclusion criteria were any lifetime axis I or II psychiatric disorders and any past or current psychopharmacological medication. To assess a possible history of abuse and neglect, we administered the Childhood Trauma Questionnaire (CTQ; Bernstein et al., Reference Bernstein, Fink, Handelsman, Foote, Lovejoy, Wenzel and Ruggiero1994). General attitude toward touch was assessed using a Social Touch Questionnaire (STQ; Wilhelm, Kochar, Roth, & Gross, Reference Wilhelm, Kochar, Roth and Gross2001) and trait anxiety was measured using the State-Trait Anxiety Inventory (STAI; Spielberger, Gorsuch, Lushene, Vagg, & Jacobs, Reference Spielberger, Gorsuch, Lushene, Vagg and Jacobs1983).
Table 1. Demographic and clinical data for patients and controls

Abbreviations: BDI-II, Beck Depression Inventory; HDRS, 17-item Hamilton Depression Rating Scale; STAI, State-Trait Anxiety Inventory.
Values are given as frequencies or as means (s.d.). The p values report the significance levels reached for independent t tests or Fisher's exact tests comparing groups or for paired t tests comparing improvement within patients.
1 BDI-II Follow-up measurements are compared to baseline scores. The significance threshold was set at p < 0.05.
Patients underwent MRI scanning within 1–3 days after admission to the clinic and, again, 24 days later; accordingly, controls were examined twice at the same interval. For the duration of the study, patients received treatment according to current guidelines for MDD (DGPPN, BÄK, KBV, & AWMF, 2015; cf. online Supplementary information). To quantify clinical improvement, trained raters assessed depressive symptom severity on a weekly basis using the 17-item Hamilton Depression Rating Scale (HDRS-17; Hamilton, Reference Hamilton1960). As a measure of self-assessed depression severity, the Beck Depression Inventory (BDI-II; Beck, Steer, Ball, & Ranieri, Reference Beck, Steer, Ball and Ranieri1996) was administered before and after the treatment course and every four weeks over a 12-week follow-up period.
Social touch paradigm
For the fMRI scans, we employed an adapted version of an established paradigm (Maier et al., Reference Maier, Gieling, Heinen-Ludwig, Stefan, Schultz, Güntürkün and Scheele2019; McGlone et al., Reference McGlone, Olausson, Boyle, Jones-Gotman, Dancer, Guest and Essick2012), in which tactile stimulation was manually applied to participants at different speed levels. Stimulation was administered by an experimenter who performed vertical strokes with cotton gloved hands over 20-cm zones on the participants' shins that were marked prior to the fMRI scan. During the 4-s touch, the complete zone was covered either with a single stroke at a speed of 5 cm/s (slow, affective touch) or with four repeated strokes at a speed of 20 cm/s (fast, discriminative touch). Slow is experienced as more pleasant than fast touch (Löken, Wessberg, Morrison, McGlone, & Olausson, Reference Löken, Wessberg, Morrison, McGlone and Olausson2009) and specifically elicits responses by CT afferents, which are associated with rewarding properties of touch (McGlone et al., Reference McGlone, Wessberg and Olausson2014). The experimenter was trained to keep stimulation pressure constant at both speed levels and received audio cues via headphones during the experiment to ensure constant stimulation velocity. No stimulation occurred during the no touch control condition. Each condition was repeated 20 times in randomized order. Each trial was initiated with the presentation of a white fixation cross (3 s). Fast and slow touch trials were then announced by the color of the fixation cross changing to blue (1 s). After each trial, the participant rated the comfort of the tactile stimulation on a 100-point visual analog scale that ranged from not at all comfortable (0) to very comfortable (100) and was presented for a maximum of 5 s. To minimize context effects, participants were not informed about the identity of the person administering the stimulation and the opening of the scanner was covered with a blanket during the experiment.
MRI data acquisition
Functional and structural MRI data were acquired on a 1.5 T Siemens Avanto MRI system (Siemens, Erlangen, Germany) equipped with a 12-channel standard head coil at the Life & Brain Centre, Bonn, Germany. T2*-weighted gradient-echo planar images images with blood-oxygen-level-dependent contrast were acquired during the social touch task (voxel size = 3 × 3 × 3 mm; TR = 3000 ms; TE = 50 ms; flip angle = 90°; FoV = 192 mm, matrix size = 64 × 64; 35 axial slices; ascending slice order with interslice gap of 0.3 mm). The first five volumes of each functional time series were discarded to allow for T1 equilibration. Additionally, a field map (voxel size = 3 × 3 × 3 mm; TR = 460 ms; TEfast = 4.76 ms; TEslow = 9.52 ms; flip angle = 60°; matrix size = 64 × 64; 35 axial slices; interslice gap of 0.3 mm) was acquired to correct for inhomogeneities of the magnetic field during preprocessing. Subsequently, a high-resolution structural image was acquired using a T1-weighted 3D MRI sequence (voxel size = 1 × 1 × 1 mm; TR = 1660 ms; TE = 3.09 ms; flip angle = 15°; FoV = 256 mm; matrix size = 256 × 256, 160 sagittal slices).
Data analysis
Data analyses focused on the comparison of patients with healthy controls, and on differences between those patients who responded (responders) and those who did not respond to antidepressant treatment (non-responders). The criterion for clinical response was defined as a ⩾50% reduction in HDRS-17 scores.
The fMRI data were preprocessed and analyzed using SPM12 software (Wellcome Trust Center for Neuroimaging, London, UK; http://www.fil.ion.ucl.ac.uk/spm) running in MATLAB R2010b (The MathWorks, Natick, MA). The functional data were realigned, initially to the first image in the time series, then to the mean of all images, and unwarped using the field map data. They were then coregistered to the anatomical volume acquired pre-treatment and normalized based on probabilistic tissue segmentation into 2-mm stereotaxic Montreal Neurological Institute (MNI) space. Subsequently, the images were smoothed using a 4-mm full width at half maximum Gaussian kernel. Two patients and one control had to be excluded from further fMRI analysis due to excessive head movement (>3 mm or °) during data acquisition. This resulted in a sample size of 51 patients and 40 controls. A two-level random effects approach based on the general linear model as implemented in SPM12 was used for statistical analysis. After preprocessing, conditions based on combinations of stimulus (fast touch, slow touch) and time (pre-treatment, post-treatment) were entered into a GLM for each participant together with a constant term and six realignment parameters per session to account for subject motion. On the first level, we subtracted the respective no touch control regressor from the experimental regressors for each participant and condition. On the second level, we conducted two separate analyses of variance (ANOVA) to compare patients with controls, and responders with non-responders. For each analysis, we entered the first level contrasts in separate flexible factorial models to compute the within-subject main effects of speed (fast touch, slow touch) and time (pre-treatment, post-treatment), the between-subjects main effects of group (patients, controls) or response (responders, non-responders), and their respective interactions. For each analysis, we used multiple models to partition variance in SPM as recommended when using group-level repeated measurement designs (McFarquhar, Reference McFarquhar2019).
To validate the effect of the social touch paradigm, we performed a whole-brain analysis of the control group with an initial height threshold of p < 0.001. Peak-level p values were then family-wise error (FWE) corrected for multiple comparisons and p < 0.05 was considered significant.
The main analysis focused on a set of bilateral a priori defined regions of interest consisting of the nucleus accumbens, caudate nucleus, putamen and anterior and posterior insula. These regions were defined based on the automated anatomical labeling atlas 3 (Rolls, Huang, Lin, Feng, & Joliot, Reference Rolls, Huang, Lin, Feng and Joliot2020). The peak-level threshold for significance was set to p < 0.05, FWE-corrected for multiple comparisons based on the size of each region of interest.
Behavioral data were analyzed using SPSS Statistics Version 27 (IBM Corp., Armonk, NY, USA) and all tests were two-tailed. To test for clinical improvement, a repeated measures ANOVA was performed for HDRS-17 ratings. In line with the fMRI analyses, we conducted separate mixed-design ANOVAs of social touch comfort ratings with touch speed (slow, fast) and time (pre-treatment, post-treatment) as within-subject factors and either group (patients, controls) or response (responders, non-responders) as a between-subjects factor to compare patients with controls or responders with non-responders, respectively. The threshold for significance was set to p < 0.05, and p values were Bonferroni-adjusted if appropriate (pcorr). Greenhouse-Geisser correction was applied in cases of lack of sphericity. A moderation analysis was conducted to examine the effect of potential confounders (age, sex, CTQ scores) on our analyses (cf. online Supplementary information). Partial eta-squared and Cohen's d were calculated as measures of effect size.
Results
Behavioral results
Analysis of HDRS-17 scores (shown in Fig. 1) showed a significant reduction over time (F (2.59, 134.89) = 36.82, p < 0.001, η p2 = 0.42) in patients, 23 (43.4%) of whom met the criterion for a clinical response.

Figure 1. Depression symptom severity as measured by Hamilton Depression Rating Scale (HDRS-17) scores decreased over the treatment course (a). Patients rated fast but not slow touch as significantly less comfortable than controls (b). At baseline patients reported a higher aversion to social touch than controls (c). Indicated p values are Bonferroni corrected. Violin plots are kernel density plots comparable to histograms with infinitely small bin sizes. The ribbon and error bars indicate 95%-confidence intervals. Abbreviations: CTRL, controls; PAT, patients; VAS, visual analog scale. *p < 0.05, ***p < 0.001.
Analysis of social touch comfort ratings revealed main effects of speed (F (1, 92) = 99.46, p < 0.001, η p2 = 0.52) and group (F (1, 92) = 7.12, p = 0.009, η p2 = 0.07, shown in Fig. 1). As expected, comfort ratings were higher after slow, affective touch than after fast, discriminative touch. Patients overall rated social touch as less comfortable than control participants, particularly after fast (t (92) = 3.06, pcorr = 0.012, d = 0.64) but not slow touch (t (92) = 0.79, pcorr > 0.999, d = 0.16).
The analysis comparing responders and non-responders also revealed a significant main effect of speed (F (1, 51) = 70.86, p < 0.001, η p2 = 0.58) with higher comfort ratings for slow touch, but no other significant main effects or interactions.
Patients reported a higher aversion to social touch as measured by STQ scores than controls (t (89.88) = 4.89, p < 0.001, d = 0.97), while no difference was found between responders and non-responders (t (51) = 0.08, p = 0.936, d = 0.02).
fMRI results
In the control group, social touch relative to the no touch control condition revealed widespread activations in touch-processing networks at the whole-brain level including the insula, somatosensory cortex and supramarginal gyrus (Gazzola et al., Reference Gazzola, Spezio, Etzel, Castelli, Adolphs and Keysers2012) (cf. online Supplementary information, Table S1).
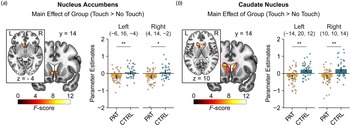
In the region of interest analysis, patients showed diminished neural response to interpersonal touch irrespective of touch velocity and time (pre v. post treatment) in the bilateral nucleus accumbens [peak MNI coordinates (x, y, z): −6, 16, −4; F (1, 89) = 15.59, p FWE = 0.010, η p2 = 0.14; MNI: 4, 14, −2; F (1, 89) = 11.68, p FWE = 0.041, η p2 = 0.11; shown in Fig. 2a] and in the bilateral caudate nucleus (MNI: − 14, 20, 12; F (1, 89) = 21.88, p FWE = 0.005, η p2 = 0.19; MNI: 10, 10, 14; F (1, 89) = 21.64, p FWE = 0.006, η p2 = 0.20; shown in Fig. 2b) compared to controls. Furthermore, we found a significant interaction between speed, time and group in the left putamen (MNI: −28, 0, 2; F (1, 89) = 19.23, p FWE = 0.016, η p2 = 0.18). Post-hoc tests revealed decreased responses to fast touch in patients compared to controls at baseline (t (89) = 3.06, pcorr = 0.036, d = 0.65) but not after treatment (t (89) = 0.38, pcorr > 0.999, d = 0.08).

Figure 2. Patients exhibited decreased neural responses to social touch in the bilateral nucleus accumbens (a) and caudate nucleus (b) across time (i.e. before and after treatment) compared with healthy controls. Significant clusters are displayed at a peak-level threshold of p < 0.05 uncorrected. Parameter estimates are displayed for peak voxels. Error bars indicate 95%-confidence intervals. Abbreviations: CTRL, controls; PAT, patients. *p < 0.05, **p < 0.01.
Secondly, we examined the effect of treatment response. The main effect of treatment response indicated reduced activity during social touch in the right caudate nucleus (MNI: 22, 20, 12; F (1, 49) = 17.86, p FWE = 0.039, η p2 = 0.26, shown in Fig. 3a) in non-responders compared to responders. A significant interaction between speed and group in the left anterior insula (MNI: −26, 26, 2; F (1, 49) = 20.01, p FWE = 0.022, η p2 = 0.30, shown in Fig. 3b) showed that non-responders exhibited reduced activation during slow touch compared to responders (t (49) = 3.75, pcorr = 0.002, d = 1.06), but not during fast touch (t (49) = 0.01, pcorr > 0.999, d < 0.01). For the interaction of speed, time and group, we found two significant clusters in the right putamen (MNI: 32, −2, −8; F (1, 49) = 19.33, p FWE = 0.032, η p2 = 0.28; MNI: 30, −6, 10; F (1, 49) = 18.20, p FWE = 0.046, η p2 = 0.27). Post-hoc tests revealed no significant effects after Bonferroni correction (all pcorr > 0.05). See online Supplementary information for main effects of time and speed.

Figure 3. Treatment responders exhibited heightened neural responses to social touch in the right caudate nucleus across time compared with non-responders (a). Responses to slow touch in the left anterior insula were increased in responders across time compared with non-responders (b). Significant clusters are displayed at a peak-level threshold of p < 0.05 uncorrected. Parameter estimates are displayed for peak voxels. Indicated p values are Bonferroni corrected. Error bars indicate 95%-confidence intervals. Abbreviations: NR, non-responders; R, responders. *p < 0.05, **p < 0.01.
The observed behavioral and neural effects of group were not significantly moderated by age or sex. We only found a significant suppressor effect of CTQ scores for the group effect on nucleus accumbens responses to social touch (cf. online Supplement).
Discussion/conclusion
To our knowledge, this is the first study to examine the processing of social touch in depression. Confirming our first hypothesis, MDD patients reported a higher aversion to interpersonal touch, experienced it as less comfortable and exhibited reduced neural activation in the reward network compared to healthy controls. Specifically, we found decreased responses to social touch in the nucleus accumbens, caudate nucleus and putamen. Contrary to our expectations, the differences in the nucleus accumbens and caudate nucleus persisted even after treatment. In line with our second hypothesis, non-responders to antidepressant treatment displayed reduced activation in the caudate nucleus, anterior insula and putamen.
Unexpectedly, patients reported decreased comfort ratings compared to controls only after fast touch. This is in line with a study that found differences in comfort ratings between participants with varying levels of childhood maltreatment during fast but not slow touch (Maier et al., Reference Maier, Gieling, Heinen-Ludwig, Stefan, Schultz, Güntürkün and Scheele2019). These findings could be related to the use of the attribute ‘comfortable’. Sailer, Hausmann, and Croy (Reference Sailer, Hausmann and Croy2020) have shown that ratings of the attributes ‘pleasant’ and ‘not burdensome’ vary with touch velocity, but a similar modulation was not evident for other emotional attributes such as ‘exciting’. In addition, possible group differences in comfort ratings after slow touch might be concealed by a ceiling effect due to high ratings in both groups. Neural effects in the nucleus accumbens and caudate nucleus were independent of touch velocity, indicating that MDD-related alterations in reward-associated brain structures are not restricted to social touch with C tactile-optimized velocity. However, in line with our hypothesis, non-responders exhibited reduced reactivity in the insula specifically during slow touch compared to responders.
These findings contribute to the notion that the processing of social reward in general (Hsu et al., Reference Hsu, Sanford, Meyers, Love, Hazlett, Walker and Zubieta2015; Laurent & Ablow, Reference Laurent and Ablow2012; Olino et al., Reference Olino, Silk, Osterritter and Forbes2015) and of interpersonal touch in particular is altered in MDD patients. Similar to patients with autism spectrum disorder who derive less pleasure from and engage in touch less frequently than healthy controls (Croy, Geide, Paulus, Weidner, & Olausson, Reference Croy, Geide, Paulus, Weidner and Olausson2016), the reported aversion to social touch in everyday life and altered reward-associated responses to social touch might relate to the emergence and reinforcement of social isolation in depression. MDD patients typically withdraw from their social circles, thus leading to smaller social network size (Elmer & Stadtfeld, Reference Elmer and Stadtfeld2020; Visentini, Cassidy, Bird, & Priebe, Reference Visentini, Cassidy, Bird and Priebe2018) and increased loneliness (Achterbergh et al., Reference Achterbergh, Pitman, Birken, Pearce, Sno and Johnson2020; Meltzer et al., Reference Meltzer, Bebbington, Dennis, Jenkins, McManus and Brugha2013), which is associated with more severe symptoms and a worse prognosis (Holvast et al., Reference Holvast, Burger, de Waal, van Marwijk, Comijs and Verhaak2015; Wang, Mann, Lloyd-Evans, Ma, & Johnson, Reference Wang, Mann, Lloyd-Evans, Ma and Johnson2018). This disruption of social functioning can have devastating consequences, as both social isolation and loss of social support have been linked to suicidal outcomes (Calati et al., Reference Calati, Ferrari, Brittner, Oasi, Olié, Carvalho and Courtet2019; Kim & Kihl, Reference Kim and Kihl2021). However, we cannot conclusively infer from reduced striatal activation that social touch is less rewarding in MDD (Poldrack, Reference Poldrack2006). For instance, striatal activation may also reflect cognitive biases or the salience of social touch. Future studies are warranted to decipher the specific mechanisms that result in decreased comfort ratings of social touch.
Interpersonal touch is a crucial component of romantic relationships (Jakubiak & Feeney, Reference Jakubiak and Feeney2017). Altered processing of social touch might blunt the drive to seek physical closeness or even result in an avoidance of interpersonal touch, which could negatively affect sexuality and the overall satisfaction in romantic relationships (Bell, Daly, & Gonzalez, Reference Bell, Daly and Gonzalez1987; Gulledge, Gulledge, & Stahmannn, Reference Gulledge, Gulledge and Stahmannn2003; Muise, Giang, & Impett, Reference Muise, Giang and Impett2014). Eventually, this might lead to separation, which is again a predictor for worse illness trajectories (Law & Sbarra, Reference Law and Sbarra2009; Woods et al., Reference Woods, Solomonov, Liles, Guillod, Kales and Sirey2021) and increased risk for suicidal behaviors (Calati et al., Reference Calati, Ferrari, Brittner, Oasi, Olié, Carvalho and Courtet2019).
Notably, the observed alterations of activity in the nucleus accumbens and caudate nucleus did not change over the treatment course. This could suggest a stable, phenotypical trait characterizing MDD patients that persists even after clinical improvement. This is in line with observations in remitted MDD patients who exhibit lasting impairments both in behavioral (Pechtel, Dutra, Goetz, & Pizzagalli, Reference Pechtel, Dutra, Goetz and Pizzagalli2013; Weinberg & Shankman, Reference Weinberg and Shankman2017) and neural markers of reward processing (Dichter, Kozink, McClernon, & Smoski, Reference Dichter, Kozink, McClernon and Smoski2012; Geugies et al., Reference Geugies, Mocking, Figueroa, Groot, Marsman, Servaas and Ruhé2019; McCabe, Cowen, & Harmer, Reference McCabe, Cowen and Harmer2009), consistent with the persistence of anhedonia even after recovery from depression (Conradi, Ormel, & de Jonge, Reference Conradi, Ormel and de Jonge2011; Schrader, Reference Schrader1997). Another explanation for the persistence of these alterations might be the relatively short time between the two fMRI sessions. While depressive symptoms went down by 40.4% across participants, a longer observation period perhaps would have allowed for further clinical improvement and behavioral adaptations. Likewise, more pronounced alterations for slow touch were only evident in non-responders to treatment.
Considering the effect of response, we found reduced caudate nucleus and insula activation during social touch in non-responders both before and after treatment, indicating that those who show greater alterations in striatal and insular reward processing might be less responsive to established antidepressant treatment, both in terms of clinical recovery and normalization of altered processing of social rewards. In the light of the devastating consequences that can arise from social isolation, this emphasizes the need for targeted interventions that focus on reward processing deficits. For instance, behavioral activation therapy (Hopko, Lejuez, Ruggiero, & Eifert, Reference Hopko, Lejuez, Ruggiero and Eifert2003) has been shown to be effective in the treatment of depression (Luoto et al., Reference Luoto, Lindholm, Paavonen, Koivukangas, Lassila, Leinonen and Kampman2018) and seems to affect striatal responses (Dichter et al., Reference Dichter, Felder, Petty, Bizzell, Ernst and Smoski2009). Furthermore, body-based interventions in the form of massage therapy (Arnold, Müller-Oerlinghausen, Hemrich, & Bönsch, Reference Arnold, Müller-Oerlinghausen, Hemrich and Bönsch2020) and body psychotherapy (Röhricht, Papadopoulos, & Priebe, Reference Röhricht, Papadopoulos and Priebe2013) are promising approaches to specifically target disturbed body awareness and desynchronization in depression (Fuchs, Reference Fuchs2001; Fuchs & Schlimme, Reference Fuchs and Schlimme2009).
Our findings should be interpreted in light of some limitations. While reward network activation during touch is in line with studies in healthy controls using various kinds of social touch conditions (Boehme et al., Reference Boehme, Hauser, Gerling, Heilig and Olausson2019; Nummenmaa et al., Reference Nummenmaa, Tuominen, Dunbar, Hirvonen, Manninen, Arponen and Sams2016; Scheele et al., Reference Scheele, Kendrick, Khouri, Kretzer, Schläpfer, Stoffel-Wagner and Hurlemann2014; Zimmermann et al., Reference Zimmermann, Kendrick, Scheele, Dau, Banger, Maier and Becker2019), other studies did not find activation of reward-related brain regions during social touch suggesting that the rewarding effects of social touch paradigms are not unambiguous (e.g. Lamm, Silani, & Singer, Reference Lamm, Silani and Singer2015). Because it is hard to dispute that an embrace from a loved one or the caresses of a romantic partner can be perceived as rewarding, this raises the question of the ecological validity of social touch paradigms, particularly in fMRI studies. While it is challenging to implement paradigms that model social rewards more accurately, previous studies examined social touch in close friends or romantic couples to increase ecological validity (Flores, Alarcón, Eckstrand, Lindenmuth, & Forbes, Reference Flores, Alarcón, Eckstrand, Lindenmuth and Forbes2022; Kreuder et al., Reference Kreuder, Scheele, Wassermann, Wollseifer, Stoffel-Wagner, Lee and Hurlemann2017; Nummenmaa et al., Reference Nummenmaa, Tuominen, Dunbar, Hirvonen, Manninen, Arponen and Sams2016). High experimental standardization can be retained using cover stories (Kreuder et al., Reference Kreuder, Scheele, Wassermann, Wollseifer, Stoffel-Wagner, Lee and Hurlemann2017). Because our current findings were acquired in a highly standardized MRI setting, which might be anxiety-inducing especially for MDD patients, they should be validated by future studies using more naturalistic social touch paradigms. Future studies should also address a number of questions to aid contextualization of our findings: firstly, future research should ask participants to specifically rate reward in addition to comfort after receiving social touch, to gain a more multifaceted picture of participants' subjective experience; secondly, control conditions should be employed to explore whether the observed alterations in MDD are specific to social touch or extend to the processing of non-social tactile stimulation; and thirdly, future studies should also examine the impact of MDD on the processing of social touch in other brain regions associated with social touch and mental disorders, such as the superior temporal gyrus (Davidovic, Jönsson, Olausson, & Björnsdotter, Reference Davidovic, Jönsson, Olausson and Björnsdotter2016; Strauss et al., Reference Strauss, Rottstädt, Sailer, Schellong, Hamilton, Raue and Croy2019). Finally, antidepressant treatment in this study was naturalistic and heterogeneous, and its particular influence on our findings therefore remains uncertain. However, the treatment was in line with current guidelines for the therapy of depression reflecting clinical realities.
In conclusion, our findings elucidate the role of social touch processing in depression and indicate that touch-related changes may persist even after significant improvements of other symptoms. Collectively, our results demonstrate alterations of the experienced comfort of and neural response to social touch in patients with MDD. Moreover, these effects may constitute a risk factor for non-response and may persist even after recovery, leading to ongoing disruptions in social functioning. Future studies should corroborate these findings and might inform new treatment avenues targeting social reward and disturbances of body awareness.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291723001617
Acknowledgements
The authors thank Paul Jung for his programming assistance as well as Anna Metzner, Lara Graute and Lea Köster for their help with data acquisition.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.