The anticonvulsants carbamazepine and sodium valproate are widely used in psychiatric practice. Carbamazepine is officially licensed for use in epilepsy, trigeminal neuralgia and for the prophylaxis of manic-depressive psychosis in patients unresponsive to lithium (Association of the British Pharmaceutical Industry, 1998). Valproate has a less broad licence and may legally be prescribed only for a variety of seizure disorders (Association of the British Pharmaceutical Industry, 1998). Both drugs may in practice, however, be prescribed for many of other conditions including mania and aggression. We sought to investigate off-licence use in a prescription and case note review.
Carbamazepine and valproate prescribing can be problematical because of the need for blood monitoring of plasma levels and of certain adverse effect parameters. So, alongside the primary investigation, we also undertook to evaluate the quality of prescribing and monitoring of the use of these drugs when set against officially recognised standards and other published recommendations.
The study
Data were collected during a predetermined week in March 1998 by pharmacists employed in 25 secondary-care psychiatry units in south-east England. Prescription cards and case notes were examined for all in-patients admitted to hospital for the treatment of mental illness prescribed either carbamazepine or valproate. Patients' age, gender and diagnosis were taken from case notes and the reasons given for prescribing either drug recorded verbatim. Pathology report forms were searched for records of plasma level monitoring and full blood counts. Drug doses and formulations were noted from prescription charts.
Findings
We found prescribing details for 292 patients on carbamazepine and 141 on valproate. The age of those on carbamazepine ranged from 17-97 years (mean 46.7) and 54.4% were male. For patients taking valproate, 49.6% were male and age ranged from 19-80 years (mean 42.5).
Indications
There were 23 recorded indications for the prescribing of carbamazepine and 13 recorded indications for prescribing sodium valproate. These were condensed into four broad indications.
-
(a) Mood disorder, including reasons stated as: mood stabiliser, bipolar affective disorder, hypomania, depression, seasonal affective disorder, schizoaffective disorder, antidepressant augmentation, affective and schizoaffective disorders.
-
(b) Seizure disorder, including reason stated as: epilepsy, mood stabiliser/epilepsy, schizophrenia and epilepsy, behaviour/epilepsy and epilepsy with aggression.
-
(c) Behavioural disturbance, including reason stated as: behavioural control, aggression and agitation.
-
(d) Other indications, including reason stated as: schizophrenia, psychosis, dementia, anorexia, antipsychotic augmentation, drug-induced psychosis and post-ictal psychosis.
The relative percentages of patients prescribed the drugs for each indication are shown in Table 1.
Table 1. Indications for prescribing, plasma level validity and full blood counts (FBCs)
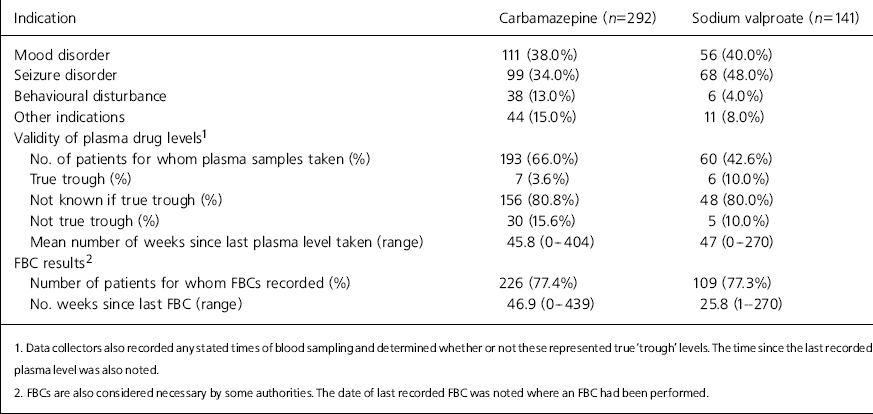
| Indication | Carbamazepine (n=292) | Sodium valproate (n=141) | ||
|---|---|---|---|---|
| Mood disorder | 111 (38.0%) | 56 (40.0%) | ||
| Seizure disorder | 99 (34.0%) | 68 (48.0%) | ||
| Behavioural disturbance | 38 (13.0%) | 6 (4.0%) | ||
| Other indications | 44 (15.0%) | 11 (8.0%) | ||
| Validity of plasma drug levels1 | ||||
| No. of patients for whom plasma samples taken (%) | 193 (66.0%) | 60 (42.6%) | ||
| True trough (%) | 7 (3.6%) | 6 (10.0%) | ||
| Not known if true trough (%) | 156 (80.8%) | 48 (80.0%) | ||
| Not true trough (%) | 30 (15.6%) | 5 (10.0%) | ||
| Mean number of weeks since last plasma level taken (range) | 45.8 (0-404) | 47 (0-270) | ||
| FBC results2 | ||||
| Number of patients for whom FBCs recorded (%) | 226 (77.4%) | 109 (77.3%) | ||
| No. weeks since last FBC (range) | 46.9 (0-439) | 25.8 (1-270) | ||
| 1. Data collectors also recorded any stated times of blood sampling and determined whether or not these represented true ‘trough’ levels. The time since the last recorded plasma level was also noted. | ||||
| 2. FBCs are also considered necessary by some authorities. The date of last recorded FBC was noted where an FBC had been performed. | ||||
The dose ranges, dosage forms, plasma levels and percentage of plasma levels above putative therapeutic threshold for each indication are shown in Table 2. The putative threshold for carbamazepine was taken to be > 7 mg/l for use as a mood stabiliser and >4 mg/l for the treatment of epilepsy. The putative threshold for sodium valproate was taken to be > 50 mg/l for use as a mood stabiliser and for the treatment of epilepsy (see Comment for full details).
Table 2. Dose and plasma level characteristics for each indication
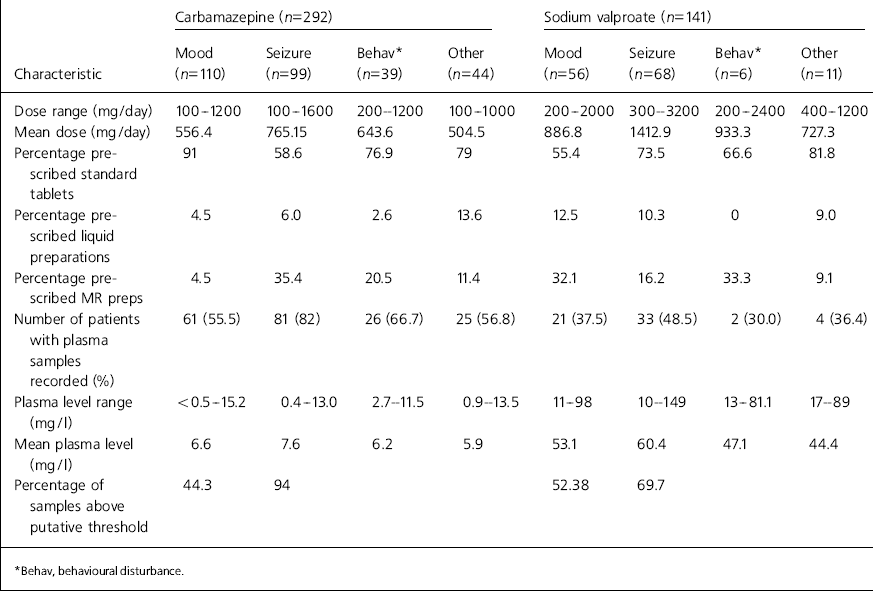
| Carbamazepine (n=292) | Sodium valproate (n=141) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Mood (n=110) | Seizure (n=99) | Behav* (n=39) | Other (n=44) | Mood (n=56) | Seizure (n=68) | Behav* (n=6) | Other (n=11) | ||||||||
| Dose range (mg/day) | 100-1200 | 100-1600 | 200-1200 | 100-1000 | 200-2000 | 300-3200 | 200-2400 | 400-1200 | ||||||||
| Mean dose (mg/day) | 556.4 | 765.15 | 643.6 | 504.5 | 886.8 | 1412.9 | 933.3 | 727.3 | ||||||||
| Percentage prescribed standard tablets | 91 | 58.6 | 76.9 | 79 | 55.4 | 73.5 | 66.6 | 81.8 | ||||||||
| Percentage prescribed liquid preparations | 4.5 | 6.0 | 2.6 | 13.6 | 12.5 | 10.3 | 0 | 9.0 | ||||||||
| Percentage prescribed MR preps | 4.5 | 35.4 | 20.5 | 11.4 | 32.1 | 16.2 | 33.3 | 9.1 | ||||||||
| Number of patients with plasma samples recorded (%) | 61 (55.5) | 81 (82) | 26 (66.7) | 25 (56.8) | 21 (37.5) | 33 (48.5) | 2 (30.0) | 4 (36.4) | ||||||||
| Plasma level range (mg/l) | <0.5-15.2 | 0.4-13.0 | 2.7-11.5 | 0.9-13.5 | 11-98 | 10-149 | 13-81.1 | 17-89 | ||||||||
| Mean plasma level (mg/l) | 6.6 | 7.6 | 6.2 | 5.9 | 53.1 | 60.4 | 47.1 | 44.4 | ||||||||
| Percentage of samples above putative threshold | 44.3 | 94 | 52.38 | 69.7 | ||||||||||||
| * Behav, behavioural disturbance. | ||||||||||||||||
Comment
We surveyed treatment aspects of more than 400 mental health patients prescribed carbamazepine or valproate. A wide variety of reasons for prescribing these drugs were recorded in case notes. For carbamazepine, 34% of prescribing was for seizure disorders and 38% for mood disorders, according to our classification. Most of this prescribing could be described as on-licence, although this was not entirely clear for many mood disorders recorded and, in addition, refractoriness to lithium was not always noted. Thus, at least 28% of carbamazepine prescribing was off-licence in this study. For valproate, at least 52% of prescribing was apparently for indications not listed in the drug's product licence. Prescribers should be aware of the potential legal consequences of adverse effects resulting from off-licence use.
The quality of carbamazepine and valproate monitoring is difficult to assess, because, for many indications, clear standards of monitoring are absent. However, most authorities would agree that plasma drug level monitoring is mandatory when using carbamazepine for seizure disorders (Reference EadieEadie, 1995; Association of the British Pharmaceutical Industry, 1998) and at least helpful for valproate in seizure disorders (Reference Balfour and BrysonBalfour & Bryson, 1994) and for both drugs in bipolar disorder (Reference Taylor and DuncanTaylor & Duncan, 1997; Reference Taylor, Duncan, McConnell and McConnellTaylor et al, 1999). Given these observations, the proportion of patients in this study for whom no plasma levels had been measured is concerning. Added to this is the finding that, with the exception of carbamazepine in seizure disorders, the proportion of patients with ‘therapeutic’ plasma levels was surprisingly low, especially in mood disorder. For the purposes of this study therapeutic plasma level thresholds were assumed to be 4 mg/l for carbamazepine in seizure disorder (Reference ReynoldsReynolds, 1978; Association of the British Pharmaceutical Industry, 1998); 8 mg/l for carbamazepine in bipolar disorder (Reference Taylor and DuncanTaylor & Duncan, 1997), and 50 mg/l for valproate in either condition (Reference Taylor, Duncan, McConnell and McConnellTaylor et al, 1999; Reference Balfour and BrysonBalfour & Bryson, 1994). There are apparently no clear target levels in other disorders.
Even more concerning is the apparent poor quality of sampling and record keeping. Sampling of both drugs should take place at the end of the dosing interval, so as to give a ‘trough’ level on which quoted therapeutic ranges and thresholds are based. In our sample, it could not be established that a true trough sample had been taken in more than 80% of cases. In fact, less than 10% were clearly true trough levels. These findings have a potentially major impact on the interpretation of blood drug levels and may even lead to inappropriate dose changes. Also, the frequency of drug level monitoring was low on average, substantially lower than recommended frequencies, even in long-term therapy (Reference Taylor, Duncan, McConnell and McConnellTaylor et al, 1999). Nevertheless, the mean values cited here were calculated including those for whom no sample had been taken (possibly appropriate) and thus may not be an accurate reflection of sample frequency in appropriate subjects.
We also examined the use of full blood counts used to monitor possible drug toxicity. Carbamazepine is associated with neutropenia, agranulocytosis and aplastic anaemia (Association of the British Pharmaceutical Industry, 1998; Reference Cates and PowersCates & Powers, 1998). Valproate can cause thrombocytopenia and, more rarely, leucopenia and pancytopenia (Reference Balfour and BrysonBalfour & Bryson, 1994). Blood monitoring is therefore essential for both drugs. We found that full blood counts had been performed for just over three-quarters of patients, but that monitoring was infrequent. Many patients had not had counts performed for some years.
Our findings concur with studies in other, similar fields. For example, in epilepsy, anticonvulsant monitoring is strongly recommended, but often poorly done (Reference Schoenenberger, Tanasijevic and JhaSchoenenberger et al, 1995). In mental health patients, clear, unequivocal standards for the use of carbamazepine and valproate have not been established. However, our study has shown that, where clear guidance on prescribing is available, prescribing and monitoring often fall short of what might be considered acceptable practice. Prescribers and pharmacists need to be better aware of published guidance and to follow more closely recommendations made. Our findings also support the need for proposed clinical governance initiatives relating to the quality of prescribing.





eLetters
No eLetters have been published for this article.