Buprenorphine is a semi-synthetic derivative of opium which was licensed in the UK in 2001 for the treatment of opiate dependence. Many studies have suggested that buprenorphine is a safe and efficacious treatment (Reference Ling and WessonLing & Wesson, 2003; Reference Gowing, Ali and WhiteGowing et al, 2006), and some have suggested advantages over methadone, resulting in part from the partial agonist and antagonist receptor profile. This profile affords better safety in overdose (Reference Auriacombe, Fatseas and DubernetAuriacombe et al, 2004; Reference Luty, O'Gara and SessayLuty et al, 2005) and a lack of euphoria if heroin is also used. Fischer et al (Reference Fischer, Gombas and Eder1999) demonstrated significantly lower rates of illicit opiate consumption in opiate-dependent patients treated with buprenorphine compared with methadone maintenance.
Despite such evidence, until 3 years ago buprenorphine had not been used routinely in North Derbyshire to treat opiate dependence. This paper describes the setting up and auditing of a buprenorphine prescribing clinic within the Chesterfield Community Drug Team.
Method
A protocol was devised for prescribing buprenorphine for opiate detoxification with the help of 12 protocols from other centres and advice from the drug manufacturer. Central to this protocol (a full copy of which is available from the author) were the following exclusion criteria: age under 18 years; pregnant or breast-feeding; frequent (more than twice a week) intravenous injecting; polydrug misuse; alcohol dependence or harmful use; known hypersensitivity to buprenorphine; severe hepatic, renal or respiratory impairment; current use of more than 30 mg methadone or 0.5 g heroin per day.
A weekly prescribing clinic was set up over a 6-month period from July 2002 to January 2003, and took appropriate referrals (n=33) from keyworkers of the community drug team. About a dozen referrals were excluded from the trial after assessment. The most common reasons for exclusion were patient preference for methadone, unstable illicit drug use or regular use greater than 0.5 g heroin per day. Some of the patients included in the scheme had been newly referred to the community drug team and had no previous history of detoxification; others had had one or more previous detoxifications with methadone or lofexidine. Patients were allocated to a 16- or 30-day detoxification regime (details available from author on request) depending upon their level of opiate use, motivation and social support. Illicit opiate use following allocation was demonstrated by both instant urine drug screening and patient self-report. The process was audited using 11 standards (Table 1). Standards were devised from commonly accepted nationwide practice, as ascertained from external protocols and recommendations of the drug manufacturer.
Table 1. Audit standards for opiate detoxification with buprenorphine and achievement of standards
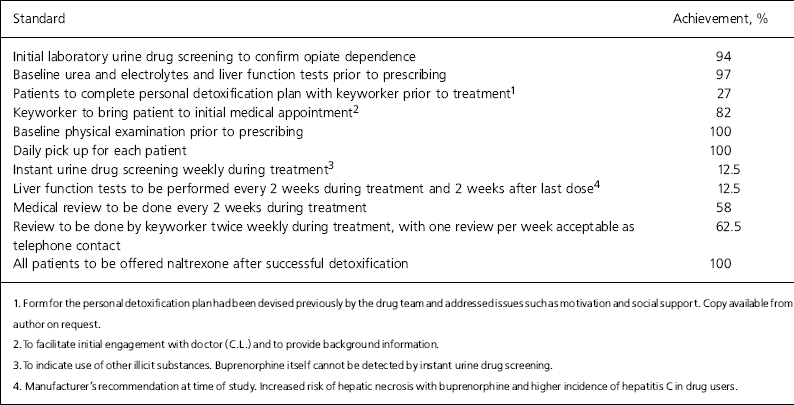
| Standard | Achievement, % |
|---|---|
| Initial laboratory urine drug screening to confirm opiate dependence | 94 |
| Baseline urea and electrolytes and liver function tests prior to prescribing | 97 |
| Patients to complete personal detoxification plan with keyworker prior to treatment1 | 27 |
| Keyworker to bring patient to initial medical appointment2 | 82 |
| Baseline physical examination prior to prescribing | 100 |
| Daily pick up for each patient | 100 |
| Instant urine drug screening weekly during treatment3 | 12.5 |
| Liver function tests to be performed every 2 weeks during treatment and 2 weeks after last dose4 | 12.5 |
| Medical review to be done every 2 weeks during treatment | 58 |
| Review to be done by keyworker twice weekly during treatment, with one review per week acceptable as telephone contact | 62.5 |
| All patients to be offered naltrexone after successful detoxification | 100 |
Two-year follow-up data were collected for selected patients and comprised: whether the patient was still in contact with the service; and if so, whether they were still receiving treatment for opiate dependence.
Results
Of the 33 patients meeting the inclusion criteria for detoxification, 9 failed to adhere to the programme from the outset, leaving 24 who underwent treatment. Of these, 7 successfully completed the detoxification and were offered naltrexone. This equates to a number needed to treat (NNT) of 5.
Those who failed to adhere from the outset either: (a) failed to pick up their buprenorphine at all and continued to use illicit opiates as previously; (b) commenced detoxification but within a few days failed to collect their buprenorphine and returned to illicit opiate use; or (c) commenced detoxification but continued to use illicit opiates as previously (i.e. in similar amounts) from the outset. Successful completion of detoxification was defined as: (a) completion of the detoxification regime and (b) absence of illicit opiate use on completion. Patients who used small amounts of illicit opiates occasionally but otherwise followed the regime were still regarded as having successfully completed detoxification.
Only 5 of the 11 audit standards were met at 94% achievement rate or greater (Table 1); 100% achievement rates were shown for ‘baseline physical examination prior to prescribing’ and ‘daily pick up for each patient’. Particularly poor achievement rates of 12.5% were seen for ‘weekly instant urine drug screening during treatment’ and ‘liver function tests to be performed twice weekly during treatment and 2 weeks after the last dose’.
Two-year follow-up data revealed no clear advantage for the 7 patients who had successfully completed detoxification, with regard to ongoing contact with the service and continued treatment for opiate dependence (Table 2). Moreover, of the 9 patients who failed to adhere to detoxification from the outset, 2 had ceased to use illicit opiates at 2-year follow-up compared with none of the 7 completing the programme.
Table 2. Two-year follow-up data
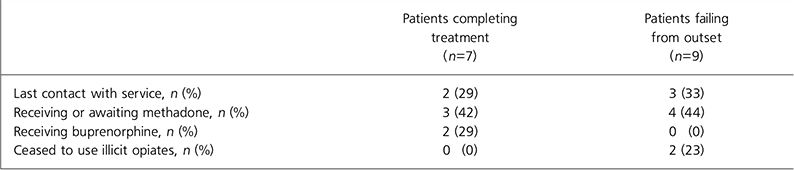
| Patients completing treatment (n=7) | Patients failing from outset (n=9) | |
|---|---|---|
| Last contact with service, n (%) | 2 (29) | 3 (33) |
| Receiving or awaiting methadone, n (%) | 3 (42) | 4 (44) |
| Receiving buprenorphine, n (%) | 2 (29) | 0 (0) |
| Ceased to use illicit opiates, n (%) | 0 (0) | 2 (23) |
Discussion
This study indicates that buprenorphine detoxification may be of benefit to some opiate-dependent patients. However, because of small numbers there was insufficient power to determine any statistically significant effect on treatment outcome of previous detoxification history, level of opiate use, etc. Therefore although encouraging in such a challenging population the NNT of 5 should be considered within this context.
Exclusion criteria included frequent intravenous injecting but it should be noted that buprenorphine can be safely used to treat injecting opiate users, provided it is consumed under direct pharmacy supervision. Although the exclusion criteria were those used by the trust for detoxification at the time of this study, buprenorphine is no more (and probably less) dangerous than methadone in intravenous opiate users. The same is also true for those with polydrug use, concurrent alcohol misuse and renal, respiratory or hepatic failure.
The audit standards reflected sound clinical practice, but there were practical problems in meeting these standards, not least because those using drugs may have particular difficulties with treatment adherence. In retrospect, standards relating to blood testing were perhaps overzealous. There are reports of minor liver dysfunction associated with buprenorphine use (Reference Ford, Morton and LintzerisFord et al, 2003), but the Committee on the Safety of Medicine does not highlight this. Moreover, the British National Formulary (http://bnf.org/bnf) does not mention liver function testing with buprenorphine prescribing. Keeping blood tests to a minimum would be advisable as many drug users have poor veins and carry the hepatitis C virus. However, the manufacturers recommend that liver function be checked at assessment and every 6 months in accordance with guidelines from the Royal College of General Practitioners (Reference Ford, Morton and LintzerisFord et al, 2003). These guidelines also state that if there is clinical evidence of liver disease, liver function tests should be performed before treatment and again at 2–3 months (Reference Petry, Bickel and PiaseckiPetry et al, 2000).
The low rate of urine testing reflected the low rate of attendance at the clinic. Training keyworkers to perform instant urine drug screening at the patients’ homes might be helpful here. It would also be reasonable to reduce the frequency of testing to every 2 weeks.
Increased staff time for administration would almost certainly have helped to increase the proportion of patients with a personal detoxification plan on file. Of the 24 patients in treatment, only 2 were known to have no personal detoxification plans, case notes were missing for 3, and 19 plans were thought to have been completed but could not be found in the notes.
There is much evidence suggesting that buprenorphine is at least as effective as methadone in maintenance treatment of opiate dependence (Reference Ling and WessonLing & Wesson, 2003). However, evidence for its effectiveness in detoxification programmes is poor (Reference LutyLuty, 2003), although Ling et al (Reference Ling, Amass and Shoptaw2005) demonstrated the benefits of buprenorphine/naltrexone v. clonidine in both in-patient and out-patient detoxification. Gowing et al (Reference Gowing, Ali and White2006) reported buprenorphine to be more effective than clonidine, and of similar effectiveness to methadone, for the management of opioid detoxification.
At the time of setting up the clinic, buprenorphine was only available within the trust for detoxification, however the trust protocol now refers to maintenance treatment. This should arguably be the way forward for the treatment of opioid dependence. However, reluctance to use buprenorphine in preference to methadone persists, despite the advantages of the former. Cost may be an issue, as buprenorphine is considerably more expensive than methadone. However, Doran et al (Reference Doran, Shanahan and Mattick2003) showed no statistically significant difference in cost-effectiveness of buprenorphine v. methadone maintenance. Buprenorphine has fewer sedative properties than methadone. Such properties may be beneficial in easing distress during treatment. Also, it should be noted that supervision of consumption by a pharmacist takes longer with buprenorphine than with methadone. None the less the case for buprenorphine as a first-line treatment for opioid dependence, at least with respect to maintenance, remains strong and should be given careful consideration.
Declaration of interest
None.
Acknowledgements
Thanks to Dr J. G. Cunnane (Consultant Psychiatrist, Doncaster Royal Infirmary), the Chesterfield Community Drug Team, Professor Sean Spence (Professor of Adult Psychiatry, University of Sheffield) and Dr N. Sievewright (Consultant Psychiatrist in Substance Misuse, Sheffield).





eLetters
No eLetters have been published for this article.