Antipsychotic medication is commonly prescribed for individuals with learning disabilities. It is estimated that between 20% and 45% of this patient group are on such medication (Reference Deb and FraserDeb & Fraser, 1994). Management of behavioural problems appears to be the most common reason for the prescription of such drugs (Reference Wressell, Tyrer and BerneyWressell et al, 1990). Psychiatric indications for antipsychotic drug prescription include: schizophrenia and other psychoses, mania, motor tics, severe anxiety and psychomotor agitation, excitement, agitation and restlessness, violent or dangerously impulsive behaviour and the control of deviant antisocial sexual behaviour (British Medical Association & Royal Pharmaceutical Society of Great Britain, 2001).
A recent, randomised controlled trial has shown that a substantial proportion of people with learning disabilities who were prescribed antipsychotic medication for behavioural disorders can now potentially have their drugs reduced or withdrawn (Reference Ahmed, Fraser and KerrAhmed et al, 2000). Worsening of behavioural problems, leading to drug reinstatement, has also been reported (Reference Fielding, Murphy and ReaganFielding et al, 1980; Reference BriggsBriggs, 1989).
Thioridazine is an antipsychotic drug belonging to the phenothiazine group. Historically, within South Wales, it has commonly been prescribed among the group of patients with learning disabilities. On 12 December 2000, restricted indications and new warnings on the potential cardiotoxicity of thioridazine were circulated by the Chief Medical Officer and Chief Pharmaceutical Adviser of the National Assembly of Wales (Chief Medical Officer, 2000). The Committee on Safety of Medicines (CSM) had considered the evidence regarding prolongation of QTc interval and life-threatening ventricular arrhythmias observed with this drug. In summary, they advised that:
-
(a) thioridazine use should be restricted to the second-line treatment of schizophrenia in adults;
-
(b) the balance of risks and benefits is unfavourable for other previous indications;
-
(c) all patients should have a baseline electrocardiogram (ECG) and electrolytes, which should be repeated after a dose escalation and at 6-monthly intervals;
-
(d) thioridazine prescription should be re-evaluated and in cases of discontinuation, a gradual dose reduction over 1-2 weeks is recommended.
Following the CSM warning, a study was designed to describe the use of thioridazine in a population of adults with learning disabilities within Bro Morgannwg NHS Trust, South Wales. Other aims were to assess the effects of drug discontinuation and establish those factors that were linked to substantial problems in this patient group.
Method
The study was carried out within the Learning Disability Directorate of Bro Morgannwg NHS Trust, South Wales. (The Directorate provides services for a large catchment area, serving a population of approximately 1.3 million.) Individuals with a learning disability were identified who were known to community learning disability teams and/or consultant learning disability psychiatrists and who were prescribed thioridazine at the time of the CSM warning. A simple questionnaire was devised to assist in information gathering (further details are available from the authors on request). Retrospective case note analysis was performed. Ambiguities were clarified, if necessary, by discussion with relevant members of the nursing staff or the community learning disability team. Two outcome measures were defined; either individuals had significant adverse events on/following thioridazine withdrawal or they did not. Operational criteria were defined to clarify what was meant by ‘adverse events’ (Box 1).
Box 1. Operational criteria to define ‘adverse events’
| During or following thioridazine drug withdrawal: |
|
A total of 187 individuals were identified as being on thioridazine. Because of time restraints, we decided to select a randomised sample of 94 patients (50%) for the study. The randomisation was performed using tables of random numbers that identified 94 individuals. From these 94 patients, three were excluded from the study (one had died, one had moved out of the area and one withdrew from thioridazine before December 2000). Statistical analysis of the 91 patients remaining in the study was performed using SPSS for Windows version 6.
Results
Of the 91 individuals included in the study, 77 were identified as being on regular thioridazine, the remaining 14 only taking it on an ‘as required’ basis. Of the 77 individuals on regular medication, the mean age was 45.5 years (standard deviation=13.4 years, range=16-81 years). Thirty-nine individuals were male and 38 female. The most common indications for prescription were for control of aggressive behaviour (n=27) and for management of symptoms of anxiety and/or agitation (n=23). Thioridazine was prescribed for other reasons in 18 individuals. These included psychoses, overactivity, restlessness, destructive behaviour, self-injury, mood disturbance and sleep disturbance. No clear indication could be found for the prescription of thioridazine in the remaining nine individuals. With respect to the degree of learning disability, 17 had a mild learning disability, 17 a moderate learning disability and 30 a severe or profound learning disability. Data were not available for the remaining 13 individuals. Forty-four individuals also had a psychiatric diagnosis and 17 had active epilepsy.
The mean daily thioridazine dose was 80 mg (median=50 mg, range=10-400 mg). Individuals had been taking thioridazine for a mean of 7.3 years (median=5.8 years, range=3 months—29 years). Thioridazine withdrawal was achieved within 2 weeks in over 50% of the group of 77 individuals. Of those 77, 59 were placed on another drug and the dose of another regularly prescribed antipsychotic was increased for four patients. It is particularly interesting to note that the most common substituted drug was chlorpromazine (n=36). Risperidone was substituted in 16 people. Other drugs used were mainly alternative atypical antipsychotics.
Individuals were described as having ‘adverse events’ if they satisfied the criteria outlined in Box 1. Full data regarding outcome were not available on all individuals. However, it was possible to say that at least 42 out of 77 individuals (55%) suffered with adverse events during or following withdrawal of thioridazine medication. Problems encountered included re-emergence of psychosis or mood disturbance, escalation of arousal, aggression, anxiety, self-injury, sexual disinhibition and ritualised behaviours.
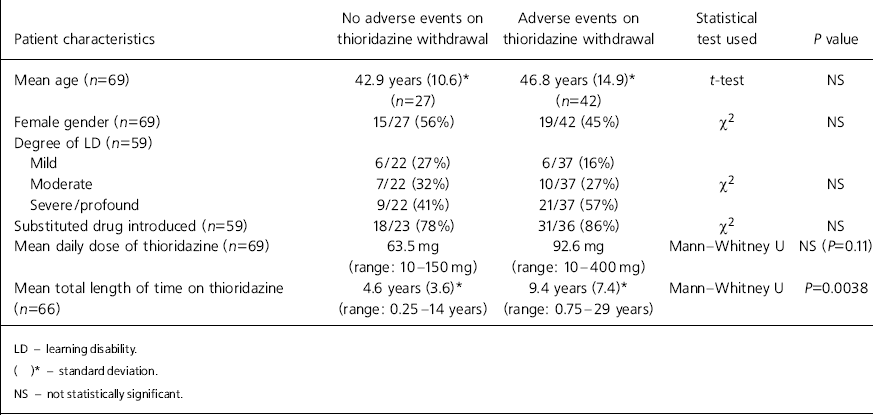
Patient characteristics for the two outcome groups are outlined in Table 1. No significant differences were apparent in the age, gender or degree of learning disability between individuals in the two outcome groups. Drug substitution was not significantly different between the two groups. Although a higher mean dose of thioridazine had been prescribed among the group who displayed problems during and after withdrawal (92.6 mg cf. 63.5 mg), this was not statistically significant. A longer mean treatment duration time was, however, significantly associated with the occurrence of adverse events (9.4 years cf. 4.6 years; P=0.0038).
Table 1. Adverse events v. patient characteristics
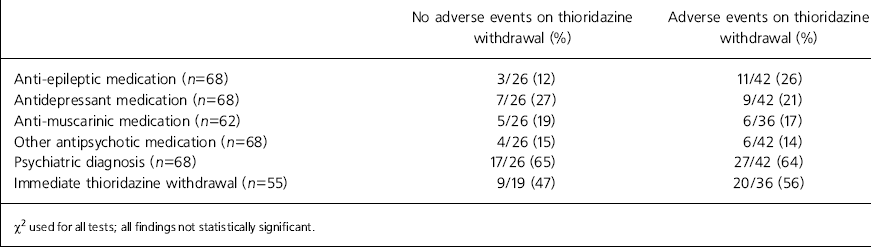
| Patient characteristics | No adverse events on thioridazine withdrawal | Adverse events on thioridazine withdrawal | Statistical test used | P value |
|---|---|---|---|---|
| Mean age (n=69) | 42.9 years (10.6)* (n=27) | 46.8 years (14.9)* (n=42) | t-test | NS |
| Female gender (n=69) | 15/27 (56%) | 19/42 (45%) | χ2 | NS |
| Degree of LD (n=59) | ||||
| Mild | 6/22 (27%) | 6/37 (16%) | ||
| Moderate | 7/22 (32%) | 10/37 (27%) | χ2 | NS |
| Severe/profound | 9/22 (41%) | 21/37 (57%) | ||
| Substituted drug introduced (n=59) | 18/23 (78%) | 31/36 (86%) | χ2 | NS |
| Mean daily dose of thioridazine (n=69) | 63.5 mg (range: 10-150 mg) | 92.6 mg (range: 10-400 mg) | Mann—Whitney U | NS (P=0.11) |
| Mean total length of time on thioridazine (n=66) | 4.6 years (3.6)* (range: 0.25-14 years) | 9.4 years (7.4)* (range: 0.75-29 years) | Mann—Whitney U | P=0.0038 |
Data regarding the prescription of other psychotropic drugs are outlined in Table 2. No association with adverse events was evident given the presence of a psychiatric diagnosis, additional psychotropic drug prescription or immediate withdrawal of thioridazine.
Table 2. Adverse events v. psychotropic drug prescription, psychiatric diagnosis and immediate thioridazine withdrawal
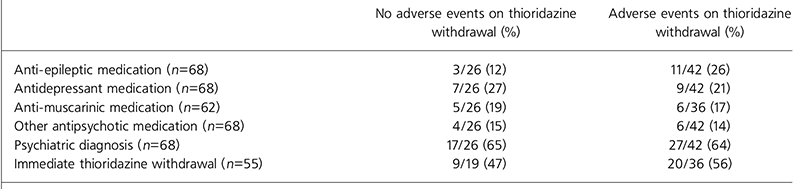
| No adverse events on thioridazine withdrawal (%) | Adverse events on thioridazine withdrawal (%) | |
|---|---|---|
| Anti-epileptic medication (n=68) | 3/26 (12) | 11/42 (26) |
| Antidepressant medication (n=68) | 7/26 (27) | 9/42 (21) |
| Anti-muscarinic medication (n=62) | 5/26 (19) | 6/36 (17) |
| Other antipsychotic medication (n=68) | 4/26 (15) | 6/42 (14) |
| Psychiatric diagnosis (n=68) | 17/26 (65) | 27/42 (64) |
| Immediate thioridazine withdrawal (n=55) | 9/19 (47) | 20/36 (56) |
Discussion
This study demonstrates that over 50% of individuals with a learning disability who were taking thioridazine at the time of the CSM warning experienced significant problems during or after withdrawal, (albeit, eight of the individuals in the study group had a history of psychosis). Re-emergence of psychosis or mood disturbance was common, as well as escalation of behavioural disturbance. Tardive dyskinesia was observed in at least four individuals. The only finding that was significantly associated with adverse events was a longer total length of time on thioridazine. However, there were also trends that suggested that a severe/profound degree of learning disability, as well as a higher mean dose of thioridazine, might be factors related to a poorer outcome. Perhaps somewhat surprisingly, a number of individuals on very small doses (< 50 mg/day) had adverse events. Although, on the whole, there is sparse literature on this subject, Branford (Reference Branford1996) found that the presence of epilepsy, lower initial antipsychotic dosage and a greater degree of learning disability were all associated with better outcomes.
The rate of thioridazine withdrawal seemed less important than the actual amount by which the dose was reduced. Although not statistically significant, the average weekly reductions of dose were 48 mg for the adverse events group (data available for n=19), versus 25 mg for the group who did not experience problems (n=11). Substitution of thioridazine with an alternative antipsychotic did not appear to affect outcome. The results here should be interpreted with caution as follow-up data were not complete for all individuals. However, for most analyses, results were available for at least 68 out of 77 individuals (88%), which is reasonable.
The findings suggest that special caution should be exercised when attempting to withdraw antipsychotic medication among individuals with a learning disability. Particular care should be exercised when withdrawing antipsychotics from individuals with a more severe degree of learning disability, those who are taking high doses and particularly those who have been on such medication for a long period of time.
Declaration of interest
None, other than that both authors were employed by Bro Morgannwg NHT Trust at the time the research was carried out.






eLetters
No eLetters have been published for this article.