In practice, typical and atypical antipscyhotics are commonly co-prescribed (Reference EreshefskyEreshefsky, 1999; Reference Taylor, Mace and MirTaylor et al, 2000a) despite clear evidence that such prescribing substantially increases the use of anticholingeric medication (Taylor et al, Reference Taylor, Mace and Mir2000a ,Reference Taylor, Mir and Kerwin b ). This increased frequency of anticholinergic prescribing is an accepted marker of an increased frequency or severity of acute extrapyramidal side-effects (EPS), presumably brought about by additional blockade of striatal dopamine D2 receptors (Reference Kapur, Roy and DaskalakisKapur et al, 2001).
Very few data support the supposed therapeutic benefits of atypical—typical co-prescribing. As far as we are aware, only one randomised controlled trial has been conducted in this field (Reference Shiloh, Zemishlany and AizenbergShiloh et al, 1997) and this showed only a modest benefit for the addition of sulpiride to clozapine therapy. Other supporting data are rather less compelling (Reference YuzdaYuzda, 2000), being derived largely from case studies or case series (e.g. Reference Mowerman and SirisMowerman & Siris, 1996).
Thus, co-prescription of atypical and typical antipsychotics continues to be widespread in the face of weak evidence of benefit and rather stronger evidence of a deleterious effect on tolerability. Little or nothing is known of the reasoning behind decisions to co-prescribe, the sequence of prescribing or of the documented outcome of co-prescribing in everyday practice.
This study was undertaken to evaluate these elements of co-prescribing of atypical and typical antipsychotics.
The study
In the first quarter of 2001 we reviewed all prescriptions written for patients of the South London and Maudsley NHS Trust. This review included all in-patients and community patients whose medication was supplied by trust pharmacies, but excluded out-patients and patients obtaining medication from community pharmacies.
All patients co-prescribed regular atypical antipsychotics with any other regular antipsychotic for longer than 6 weeks at full treatment doses were identified. For these patients, prescribing details and medication histories were recorded and patient notes examined to determine demographic data, documented reasoning for co-prescribing and documented outcome.
Findings
We examined 1441 prescriptions and uncovered 53 patients (4%) fulfilling the criteria above. Their mean age was 40.5 years (range 21-71) and 34 (64%) were male. All had a diagnosis of schizophrenia or schizoaffective disorder.
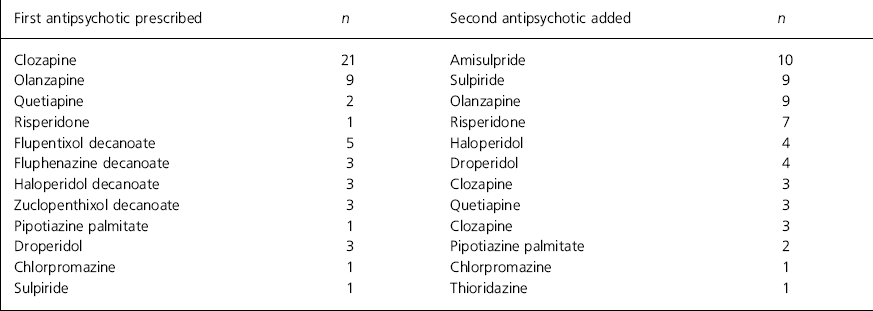
In 33 patients (62%) an atypical antipsychotic had been prescribed first and another antipsychotic added to it. Clozapine was first prescribed in 21 of these patients, olanzapine in nine, quetiapine in two and risperidone in one. For the other 20 patients (38%) a typical antipsychotic was first prescribed (depot 15, oral typical five patients) (see Table 1 for details).
Table 1. Details of first and second antipsychotic prescribed
| First antipsychotic prescribed | n | Second antipsychotic added | n |
|---|---|---|---|
| Clozapine | 21 | Amisulpride | 10 |
| Olanzapine | 9 | Sulpiride | 9 |
| Quetiapine | 2 | Olanzapine | 9 |
| Risperidone | 1 | Risperidone | 7 |
| Flupentixol decanoate | 5 | Haloperidol | 4 |
| Fluphenazine decanoate | 3 | Droperidol | 4 |
| Haloperidol decanoate | 3 | Clozapine | 3 |
| Zuclopenthixol decanoate | 3 | Quetiapine | 3 |
| Pipotiazine palmitate | 1 | Clozapine | 3 |
| Droperidol | 3 | Pipotiazine palmitate | 2 |
| Chlorpromazine | 1 | Chlorpromazine | 1 |
| Sulpiride | 1 | Thioridazine | 1 |
The reason for co-prescribing was documented for 48 (91%) patients. Documented reasons are given in Table 2.
Table 2. Documented reasons for co-prescribing

| n | % | |
|---|---|---|
| Residual symptoms on single antipsychotic | 35 | 66 |
| Adverse effects of typical drug | 5 | 9 |
| Patient request | 3 | 6 |
| Adverse effects of atypical drug | 2 | 4 |
| Non-compliance with oral medication | 2 | 4 |
| Different mechanism of action required | 1 | 2 |
Clinical outcome of co-prescribing was documented for 34 (64%). Overall, 24 patients (45%) were noted to have shown some improvement in any symptom domain. Documented evidence of improvement in psychotic symptoms (delusions, hallucinations and thought disorder) was noted in seven cases (13%). Psychotic symptoms were clearly not improved in 27 patients (51%). In one case (2%) an improvement in adverse effects was documented. For 10 patients (19%) documentation clearly indicated that no improvement of any kind had been observed.
Comment
The main findings of this study were that co-prescription was undertaken largely in an attempt to improve symptoms and that clinical outcome, where documented, was variable but unremarkable. Where response to treatment was documented, improvements in psychotic symptoms were uncommon. Indeed, most documented changes were trivial (examples included: sleep improved, more settled and less distracted).
As discussed earlier, atypical—typical co-prescription is for the most part poorly supported by published literature. However, augmentation of clozapine with sulpiride has some clinical trial backing (Reference Shiloh, Zemishlany and AizenbergShiloh et al, 1997) and attempted augmentation with any drug is probably supportable if clozapine has proved insufficiently effective alone (Reference Chong and RemingtonChong & Remington, 2000). In 21 of 53 cases examined here, another drug was added to clozapine therapy; of these, only five had had documented improvements in psychotic symptoms. In another 12 cases, typical drugs were added to existing atypical (non-clozapine) therapy, largely in an attempt to improve psychotic symptoms. Improvement in psychotic symptoms was documented in only two cases. Clozapine may have been a more appropriate choice in these cases, since only one of these 12 had previously received the drug.
In seven of 53 cases studied here, co-prescription had arisen from an apparent desire to improve adverse effects. In most cases it was clear that the intention was to withdraw the poorly tolerated drug once the second drug had been established, but this had not been done. In only one patient was there documented improvement in adverse effects — the addition of olanzapine to fluphenazine depot reduced the severity of oro-facial dyskinesia.
Documentation of clinical change was poor — outcome could not be determined for 34% of patients in the sample. Where clinical change was documented, it was limited to brief descriptions of alterations in symptoms or adverse effects. In no case was monitoring or documentation systematic and no validated rating scales of any kind had been used.
In this study, co-prescription of atypical and typical antipsychotics rarely led to documented improvement in psychotic symptoms. Augmentation of clozapine with, for example, sulpiride, is probably valid where clozapine alone has failed, but all other co-prescription should probably be avoided. Prospective, randomised evaluations of antipsychotic polypharmacy are needed.





eLetters
No eLetters have been published for this article.