The National Institute for Clinical Excellence (NICE) issued guidance on the use of atypical antipsychotic drugs in the treatment of schizophrenia in June 2002 (National Institute for Clinical Excellence, 2002). In this guidance, NICE recommended that the antipsychotic prescribed should be jointly decided upon by the prescriber and the patient, based on an informed discussion, and such a discussion should be documented in the patient’s case notes.
Some studies have been undertaken by various organisations to evaluate issues surrounding informed choice. The National Schizophrenia Fellowship (now Rethink) carried out a survey of mental health users’ views on their medication; the report, A Question of Choice, found that only 38% of users were offered a choice of medication by their doctor and 40% had no written information about the side-effects of their psychiatric medicines (National Schizophrenia Fellowship, 2000). A follow-up survey of people taking antipsychotic medication reported similar findings: only 40% were offered a choice and 50% received written information about possible side-effects (National Schizophrenia Fellowship, 2001). Another study examining the extent to which informed consent was given by long-term psychiatric in-patients found that only a fifth of patients knew the purpose of their medication and one-tenth knew about the side-effects (Reference Brown, Billcliff and MccabeBrown et al, 2001).
In this study we aimed to evaluate the extent to which patients were provided with information and involved in the choice of antipsychotic prescribed. We also set out to discover the extent to which discussion on choice was documented in case notes.
Method
Patients were recruited from eight acute adult wards over a period of 4 weeks in May 2004. The inclusion criteria were as follows:
-
• diagnosis of schizophrenia, schizoaffective disorder or psychosis-related illness;
-
• antipsychotic medication prescribed for no longer than 6 months;
-
• patient well enough to participate as judged by the nursing staff.
Participants were interviewed by means of an informal discussion with the same interviewer (B.O.). The following information was obtained from each patient during the course of the interview:
-
• form of information received on the antipsychotic the patient was prescribed (e.g. leaflet or verbal);
-
• choices of antipsychotic offered (this was partly determined by the number of antipsychotics from which they felt they could choose);
-
• level of contribution; rated by the patient as none, limited or unrestricted.
Documentation on the choice of antipsychotic, information given and persons involved at the time of discussion was sought from the medical notes. Case notes were searched for the period from a week before the patient began the antipsychotic treatment up to a week after treatment had commenced.
Results
Thirty-three patients were eligible for the study; of these, three refused to be interviewed and the remaining 30 patients with various psychotic disorders were enrolled in the study. A majority (60%) of the patients were diagnosed with schizophrenia and the remaining 40% had various psychosis-related illnesses (Table 1).
Table 1. Demographic details
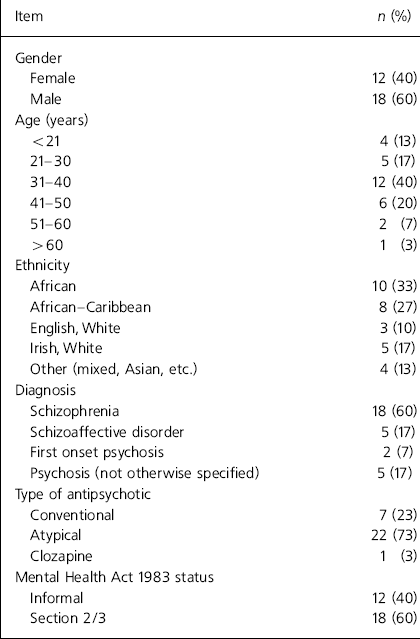
| Item | n (%) |
|---|---|
| Gender | |
| Female | 12 (40) |
| Male | 18 (60) |
| Age (years) | |
| <21 | 4 (13) |
| 21-30 | 5 (17) |
| 31-40 | 12 (40) |
| 41-50 | 6 (20) |
| 51-60 | 2 (7) |
| > 60 | 1 (3) |
| Ethnicity | |
| African | 10 (33) |
| African-Caribbean | 8 (27) |
| English, White | 3 (10) |
| Irish, White | 5 (17) |
| Other (mixed, Asian, etc.) | 4 (13) |
| Diagnosis | |
| Schizophrenia | 18 (60) |
| Schizoaffective disorder | 5 (17) |
| First onset psychosis | 2 (7) |
| Psychosis (not otherwise specified) | 5 (17) |
| Type of antipsychotic | |
| Conventional | 7 (23) |
| Atypical | 22 (73) |
| Clozapine | 1 (3) |
| Mental Health Act 1983 status | |
| Informal | 12 (40) |
| Section 2/3 | 18 (60) |
Information received
Fifteen (50%) patients claimed to have received no information at all (Table 2). One patient had refused the offer of a leaflet and seven (23%) patients received patient information leaflets. One (3%) patient could not remember if information had been received, one claimed not to care and one had received information by attending a pharmacist-led medication group session. Two (7%) patients gave responses classified as ‘other reasons’: one patient claimed he did not like to read, and the other patient received information from his wife. Two patients said they obtained information on their own from the British National Formulary and the Monthly Index of Medical Specialities.
Table 2. Patients’ responses
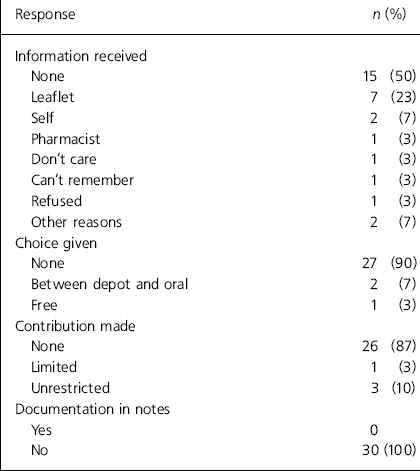
| Response | n (%) |
|---|---|
| Information received | |
| None | 15 (50) |
| Leaflet | 7 (23) |
| Self | 2 (7) |
| Pharmacist | 1 (3) |
| Don’t care | 1 (3) |
| Can’t remember | 1 (3) |
| Refused | 1 (3) |
| Other reasons | 2 (7) |
| Choice given | |
| None | 27 (90) |
| Between depot and oral | 2 (7) |
| Free | 1 (3) |
| Contribution made | |
| None | 26 (87) |
| Limited | 1 (3) |
| Unrestricted | 3 (10) |
| Documentation in notes | |
| Yes | 0 |
| No | 30 (100) |
Choice of antipsychotic
The majority of patients (27; 90%) felt they had not contributed to the choice of drug because the antipsychotic prescribed for them was chosen entirely by the prescriber or the medical team. Two (7%) patients had been offered the choice between a depot antipsychotic and an oral antipsychotic. One patient felt he had a free choice because he chose the antipsychotic he was prescribed.
Contribution to choice of antipsychotic
Three (10%) patients felt that their contribution to the choice of treatment was unrestricted. One patient felt he was partly involved, whereas 26 (87%) patients felt they were not involved at all in the decision.
Documentation in notes
None of the patients had documentation in their notes to suggest that the benefits and side-effects of their medication were discussed with them. In one case, it was documented in the notes that the offer of an antipsychotic information leaflet had been turned down by the patient.
Discussion
Our results show that the majority of patients did not feel involved in discussions about their antipsychotic medication and only a small number were given the opportunity to choose the antipsychotic prescribed by them. Also, documentation of discussions about medication in the patients’ notes was quite poor and did not appear to reflect information given by patients.
We did not examine the reasons for not allowing patients informed choice, but both patient and prescriber factors are probably relevant. For instance, nursing staff often commented that patients sometimes turned down information leaflets offered to them, possibly because of lack of insight into their illness at the time. Also, some patients were considered to be incapable of participating in an informed discussion at the point of prescribing. In such situations, NICE advises that an advocate or carer should be involved and efforts made to include them in decisions with full documentation in the notes. It is also noteworthy that there are some situations in which the choice of the antipsychotic prescribed may be determined by the nature of the illness. In treatment-resistant schizophrenia, for which clozapine is the only effective treatment, a patient might be discouraged by the lack of options.
The majority of the participants in this evaluation felt they were not given sufficient - or, in some cases, any - information about the antipsychotic prescribed. Information is sometimes withheld from patients because of concerns about non-adherence. Contrary to this belief, informing patients of the long-term side-effects of antipsychotics, such as tardive dyskinesia, seems to have little impact on adherence (Reference Chaplin and KentChaplin & Kent, 1998). A few of the patients claimed to have had to ask for an information leaflet as this was not voluntarily offered to them. Leaflets for the various antipsychotics are readily available through the trust’s intranet system which is accessible to ward staff. It remains unclear whose responsibility it is to provide these leaflets to the patients. Another area of concern is the uncertainty that the desired outcome will be achieved by providing leaflets to patients. It is assumed that information leaflets will adequately inform patients about their medication. One patient had no interest in reading the information sheet offered to him, and another could not read. Such patients and others might not benefit from reading a leaflet, but may require oral information. Whatever the reasons for our findings, there are clear implications for the training of senior house officers - those most likely to prescribe antipsychotics and with the best opportunity to discuss choice with patients.
Documentation of discussions with patients is important in this and other areas of practice. It is possible that patients interviewed in this study gave a distorted account of their contribution to drug choice. Many might have had negative feelings about their diagnosis, their leave status or their need for treatment. These feelings might have affected their recollections or reporting of events. It is also possible that the participants simply forgot discussions taking place at the stressful time of admission and first prescription. Without any entry in the case notes, it is impossible to evaluate any discrepancy between patient recall and actual events. It is recommended by NICE both that patients contribute to choice and that this contribution is recorded.
This evaluation has highlighted some interesting questions around the subject of ‘choice’: for instance, what do patients understand by choice? Often choice was interpreted as being a choice between antipsychotic treatment and no treatment at all. Only one patient was considered to have a free choice because he chose for himself the antipsychotic he was prescribed. However, it was unclear whether his choice was based on an informed discussion, because he simply asked to be given the same medication as his wife. The majority of the other patients felt that they were not offered a choice of antipsychotic. The two patients given a choice between a depot antipsychotic and oral antipsychotics were not offered the full range of antipsychotics available. Even though these patients had a choice between two antipsychotics, their choice was obviously restricted.
There are many good reasons for informing patients about medicines and for allowing them to contribute to drug choice. Not the least of these is that prescribers and patients have very different views about drug side-effects and tolerability (Reference Day, Kinderman and BentallDay et al, 1998). Moreover, involving patients in informed discussion is likely to encourage a trusting relationship between prescriber and patient and perhaps to improve adherence - patients are probably more likely to take a medicine they have chosen themselves. This is the essence of concordance - an agreement. It is not clear why prescribers are reluctant to take on this recommended practice, but further research in this area is certainly called for. In particular, a wider survey of practice would establish the generalisability or otherwise of these results obtained in a single unit.





eLetters
No eLetters have been published for this article.