The global obesity epidemic is presenting a major risk to global health. Currently, more than 1⋅9 billion adults are estimated to be obese or overweight(1). It is projected that the prevalence of obesity will double in the next 30 years, further increasing the burden of this epidemic. Obesity is associated with an increased risk of developing a range of chronic diseases such as CVD, type 2 diabetes and certain cancers(Reference Peeters, Barendregt and Willekens2). The UK National Health Service spends £6⋅1 billion on obesity-related ill-health every year, which is predicted to reach £9⋅7 billion by 2050(3). Nevertheless, an effective, non-invasive obesity treatment is yet to be found.
Current dietary and lifestyle interventions fail to provide clinically significant (5–10 %) and sustained weight loss (minimum of 1 year)(Reference Hassan, Head and Jacob4–Reference Franz, VanWormer and Crain12). The most effective weight loss strategy remains as bariatric surgery, which is an invasive procedure that may lead to undesirable side effects such as dumping syndrome and nutritional deficiencies(Reference Douketis, Macie and Thabane8, Reference Sjöström, Narbro and Sjöström13, Reference Ma and Madura14). The most commonly performed bariatric surgeries are Roux-en-Y gastric bypass, vertical sleeve gastrectomy and gastric banding which achieve 32, 25 and 20 % weight loss 1–2 years post-surgery, respectively(Reference Sjöström, Narbro and Sjöström13).
The aetiology of obesity is multifactorial and relies on the complex interaction of genetic, behavioural and environmental factors(Reference Selassie and Sinha15). This makes the identification of treatment options challenging. In principle, obesity is the result of a chronic imbalance between energy intake and energy expenditure(Reference Romieu, Dossus and Barquera16). The human body is equipped with an intricate homeostatic mechanism that works to balance energy intake and expenditure and maintain body weight(Reference Keesey and Powley17). Nevertheless, even a small but sustained positive energy balance may lead to weight gain. Using data from national surveys, Hill et al. estimated that a positive energy balance of 418·4 kJ (100 kcal)/d may be the cause of slow weight gain (0⋅5–1 kg/year) and development of obesity in US adults over recent decades(Reference Hill, Wyatt and Reed18). One factor proposed to contribute to this energy imbalance is the drastic change in diets, characterised by the increased consumption of processed foods and reduced consumption of dietary fibre(Reference Mozaffarian, Hao and Rimm19). Over past decades, diets have rapidly evolved to accommodate ‘fast foods’ or processed foods that have now become the hallmarks of western diets(Reference Kearney20, Reference Rahat-Rozenbloom, Fernandes and Gloor21). Such foods are typically energy dense and low in fibre. Accordingly, only 9 % of UK adults are estimated to meet the recommended fibre intake of 30 g/d (average intake 19 g/d)(22).
Fibre is a plant-based dietary component found in fruit, vegetables, legumes and whole grains(Reference Dhingra, Michael and Rajput23). Epidemiological studies highlight that individuals consuming high-fibre diets have lower body weights(Reference Newby, Maras and Bakun24–Reference Du, van der and Boshuizen27). This is suggested to be partly the result of an interaction between dietary fibre and the resident gut microbiota. The human gut is host to a rich, diverse and complex community of microorganisms(Reference Sender, Fuchs and Milo28). The number of gut microbes increases along the gut, with the colon having the largest population. The gut microbiota has been shown to impact its host's metabolism and confer several benefits to its host. One example is a result of its interaction with dietary fibre. Owing to its complex food structure, fibre resists digestion in the upper gut and reaches the bacteria-rich distal gut where it becomes available for bacterial fermentation(Reference Robertson, Bickerton and Dennis29). Products of bacterial fermentation have been shown to interact with the homeostatic mechanisms controlling energy metabolism. In relation to energy intake, these interactions can result in appetite suppression and reduced food intake(Reference Chambers, Viardot and Psichas30). This means that high-fibre diets may be more appetite suppressing, which may partly explain the epidemiological observations. Conversely, industrial processing of foods typically disturbs the food structures that act as barriers to digestive enzymes and results in more digestible products that are better absorbed in the upper gut. As a result, less food may reach the distal gut, thus lowering fermentation and the subsequent appetite suppression(Reference Edwards, Grundy and Grassby31–Reference Grassby, Mandalari and Grundy37). This means that, despite being more energy dense, these foods may be less appetite suppressing.
Processed foods are major components of modern diets. Understanding the relationship between food structures and appetite control may facilitate the design of foods and dietary regimens that promote appetite suppression and reduce food intake. This may provide new tools to reverse the small but sustained positive energy balance that is proposed to lead weight gain. This report reviews our current understanding of the links between food structure, the gut microbiota, with a focus on bacterial fermentation and appetite suppression.
Appetite regulation
The gastrointestinal (GI) tract is the body's largest endocrine organ and the largest interface between the human body and the external environment(Reference Ahlman and Nilsson38). This external environment includes foods and the products of digestion, and the GI tract is thus one of the key players in the body's regulation of appetite(Reference Cummings and Overduin39). The surface of the GI tract provides the means for detecting luminal nutrients and generates endocrine and neuronal signals to inform the body of their presence(Reference Helander and Fändriks40). Ultimately, these signals are transmitted to the central nervous system where they are integrated to orchestrate the short-term feelings of hunger and satiety.
Central regulation of appetite
The hypothalamus is regarded as the main ‘appetite centre’ in the central nervous system(Reference Ahima and Antwi41). Several hypothalamic regions have been shown to play a role in appetite regulation, but in particular, the arcuate nucleus (ARC) has been highlighted as important(Reference Timper and Brüning42). The ARC is strategically located near a region of the brain with an incomplete blood–brain barrier(Reference Yin and Gore43). This enables ARC to sense and integrate hormonal and metabolic signals from the peripheral circulation with the neuronal inputs from the central nervous system and periphery. The ARC contains two functionally opposing types of neurones involved in the regulation of energy balance: anorexigenic pro-opiomelanocortin (POMC) neurones and orexigenic neuropeptide Y (NPY)/agouti-related peptide (AgRP) neurones(Reference Schwartz, Woods and Porte44). Both types of neurones project to second-order neurones in other parts of hypothalamus and in extra-hypothalamic brain regions(Reference Timper and Brüning42).
Following food intake, the POMC neurones are activated(Reference Millington45). Activation of these neurones indicates the ‘fed state’ and leads to a decrease in appetite and an increase in energy expenditure(Reference Schwartz, Woods and Porte44). Conversely, the ‘fasting state’ activates orexigenic NPY/AgRP neurones(Reference Aponte, Atasoy and Sternson46). These neurons co-release NPY and AgRP which stimulate hunger and reduce energy expenditure(Reference Betley, Cao and Ritola47). Acute pharmacological activation of NPY/AgRP neurones dramatically increases energy intake in mice(Reference Krashes, Koda and Ye48). In addition, NPY/AgRP neurones directly inhibit the activity of POMC neurons in ARC through the release of inhibitory neurotransmitter γ-aminobutyric acid (Fig. 1)(Reference Cowley, Smart and Rubinstein49). The deletion of vesicular γ-aminobutyric acid transporter genes in the AgRP/NPY neurones results in a lean and obesity-resistant mouse, highlighting the physiological importance of this pathway(Reference Tong, Ye and Jones50).
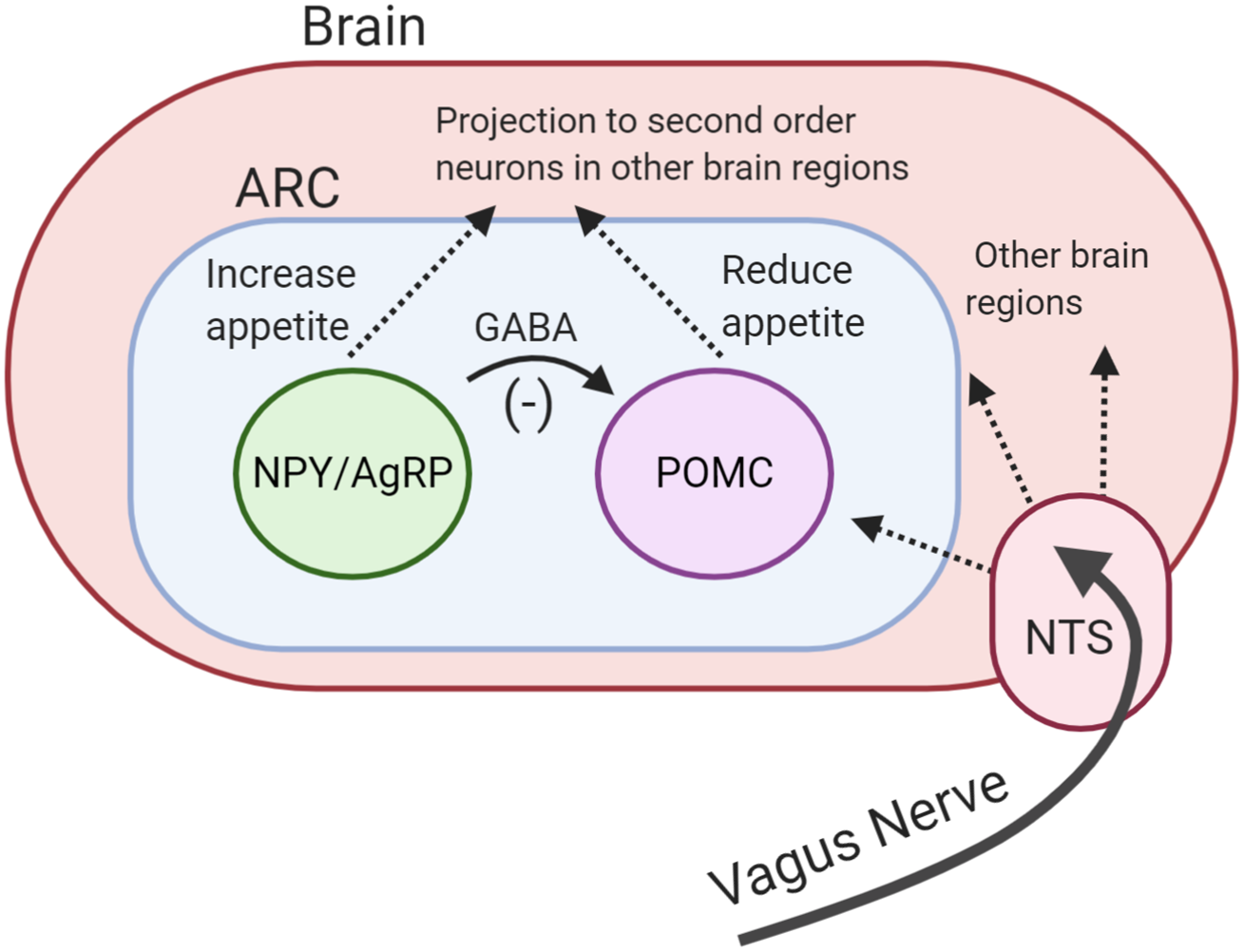
Fig. 1. (Colour online) Central regulation of appetite. Activation of pro-opiomelanocortin (POMC) neurones reduces appetite. Activation of neuropeptide Y/agouiti-related peptide (NPY/AgRP) neurones increases appetite. NPY/AgRP neurones directly inhibit the activity of POMC neurones through the release of inhibitory neurotransmitter γ-aminobutyric acid (GABA). Both neurones project to other brain regions. Vagus nerve carries signals from the periphery to the brain. ARC, arcuate nucleus; NTC, nucleus of the solitary tract.
Neuronal signals from the GI tract are transmitted to the brain via the vagus nerve. The nucleus of the solitary tract in the brainstem receives vagal afferent signals and transmits these to downstream brain regions, including the hypothalamus(Reference ter Horst, Luiten and Kuipers51). The importance of the vagus nerve has been highlighted by studies demonstrating that surgical transection of vagus nerve increases food intake and feeding duration in rodents(Reference Phillips and Powley52, Reference Schwartz53).
Peripheral control of appetite
Following food ingestion, signals are generated to increase the efficiency of digestion and reduce subsequent feeding and meal size. The entry of food in the GI tract (stomach and proximal small intestine) causes distention which stimulates mechanoreceptors(Reference Delzenne, Blundell and Brouns54). Activation of these receptors generates neuronal signals that act to slow gastric motility, allowing more time for digestion and creating a feeling of fullness(Reference Browning, Verheijden and Boeckxstaens55). In addition to this, upper GI-hormones such as cholecystokinin are released in response to the presence of food. Together, these signals are thought to provide the initial, short-lived appetite suppression.
More sustained appetite suppression is believed to be brought about by the actions of other gut hormones. The two major anorexigenic hormones implicated in appetite regulation are peptide YY (PYY) and glucagon-like peptide-1 (GLP-1)(Reference De Silva and Bloom56). Both hormones are secreted from the intestinal enteroendocrine L-cells, which express the necessary molecular machinery to sense luminal nutrients and other digestive secretions such as bile acids(Reference Spreckley and Murphy57). The density of L-cells increases along the GI tract, with the highest numbers found in the colon. In response to the detection of nutrients and other digestive factors, L-cells release GLP-1 and PYY(Reference Degen, Oesch and Casanova58, Reference Orskov, Rabenhoj and Wettergren59). All three macronutrients, their by-products and other digestive factors such as bile acids have been shown to stimulate the release of both hormones(Reference Steinert, Feinle-Bisset and Asarian60).
PYY is a peptide hormone from the pancreatic polypeptide family. There are two biologically active forms of PYY in the human body(Reference Ballantyne61). PYY (1-36) is released from L-cells and cleaved by dipeptidyl peptidase IV to form PYY (3-36). Anorectic effects of PYY (3-36) are believed to be mediated by the Y2 receptor, which is found throughout the central nervous system, including the ARC, as well as on vagal neurones(Reference Batterham, Cowley and Small62, Reference Koda, Date and Murakami63). Binding of PYY to the Y2 receptors on NPY/AgRP neurones was shown to prevent their orexigenic activity and their inhibitory effects on POMC neurones, increasing anorexigenic activity(Reference Batterham, Cowley and Small62). Increased levels of PYY decrease appetite, delay gastric emptying and reduce GI motility, contributing to the ‘ileal brake’(Reference Batterham, Cowley and Small62, Reference Batterham, Cohen and Ellis64–Reference Batterham, ffytche and Rosenthal66). Following a meal, a rise in PYY concentrations can be observed within 15 min. Given that the L-cells are mainly located in the distal gut, this early phase release is believed to be the result of neuronal stimulation or perhaps another hormone acting on L-cells, rather than reflecting the effects of direct contact of nutrients with the L-cells(Reference Suzuki, Iwasaki and Murata67). A second phase or peak of PYY is usually observed about 90 min following food intake, which is likely driven by the arrival of nutrients to the distal gut and their direct effects on L-cells(Reference Adrian, Ferri and Bacarese-Hamilton68).
GLP-1 is a peptide hormone that is also produced by intestinal L-cells, and by a population of neurones in the nucleus of the solitary tract of the brain stem. The nucleus of the solitary tract neurones are believed to be the primary source of GLP-1 in the brain, and have been shown to receive direct input from vagal afferents(Reference Holt, Richards and Cook69, Reference Hisadome, Reimann and Gribble70). The peripheral release of GLP-1 follows a similar biphasic pattern to PYY. Carbohydrate absorption in the proximal gut and other neuronal inputs are believed to contribute to its initial rise(Reference Smeets, Soenen and Luscombe-Marsh71). GLP-1 acts as an incretin hormone through binding GLP-1 receptors on pancreatic β-cells to stimulate glucose-induced insulin release(Reference Holst, Orskov and Nielsen72). GLP-1 analogues such as Exenatide and Liraglutide have been approved as diabetes treatments since 2005 and 2010, respectively(Reference Neumiller73, Reference Gupta74). GLP-1 also slows gastric emptying, inhibits glucagon secretion and suppresses appetite(Reference Tang-Christensen, Vrang and Larsen75, Reference Kreymann, Williams and Ghatei76). GLP-1 receptors are found on the vagus nerve and in the brain including the ARC(Reference Müller, Finan and Bloom77). GLP-1 infusion in the hepatic portal vein has not been shown to change the activity of POMC or NPY/AgRP neurones, suggesting intestinal GLP-1 may not directly act on the ARC but rely on vagal afferent activation to modulate the central control of appetite(Reference Müller, Finan and Bloom77, Reference Baumgartner, Pacheco-López and Rüttimann78). The pathways by which GLP-1 and PYY work to modulate the central control of appetite are still unclear.
Obese individuals have been reported to have a blunted postprandial secretion or lower fasting levels of PYY and GLP-1(Reference le Roux, Batterham and Aylwin65, Reference Carr, Larsen and Jelic79–Reference Zwirska-Korczala, Konturek and Sodowski82), which may contribute to weight gain. Bariatric surgery has also been shown to increase the postprandial release of PYY and GLP-1(Reference Svane, Jorgensen and Bojsen-Moller83). This is believed to contribute to the dramatic weight loss observed following surgery(Reference Hutch and Sandoval84). As a result, peripheral administration of GLP-1 and/or PYY has been suggested as a way to correct the low levels observed in obese individuals to encourage weight reduction, or to mimic the weight loss effects of surgery. Indeed, peripheral administration of PYY and GLP-1 has been shown to suppress appetite and reduce food intake in both rodents and lean and obese human subjects(Reference Batterham, Cowley and Small62, Reference Batterham, Cohen and Ellis64, Reference Tan, Behary and Tharakan85, Reference Verdich, Flint and Gutzwiller86). The chronic administration of these hormones also resulted in weight loss in animal and human models(Reference Batterham, Cowley and Small62, Reference Behary, Tharakan and Alexiadou87–Reference Vilsbøll, Christensen and Junker89). GLP-1 analogue Liraglutide has been approved as a weight loss treatment in patients without diabetes since 2015(Reference Dar, Tahrani and Piya90). These highlight that GLP-1 and PYY are key regulators of appetite regulation and that their manipulation may have an effect on energy intake and body weight.
Bacterial fermentation and energy metabolism
The human GI tract harbours a rich and dynamic community of microorganisms (gut microbiota) living in a symbiotic manner. Gut microbiota relies on hosts' dietary intake as an energy source to grow and multiply. The main source of energy for microbiota is a dietary fibre which becomes available for bacterial fermentation in the distal gut. The complex interplay between dietary fibre, gut microbiota and microbial-produced metabolites has an impact on hosts' energy metabolism. In relation to specific energy intake, these interactions can result in appetite suppression and reduced food intake, reducing the risk of excess energy intake and weight gain(Reference Chambers, Viardot and Psichas30).
Dietary fibre and bacterial fermentation
Dietary fibre is an umbrella term for the group of carbohydrates that cannot be digested by the endogenous enzymes in the human body. There are several definitions of dietary fibre proposed by different countries and organisations(91). The most recent, and one of the most detailed, definitions has been made by the Australia New Zealand Food Authority(92): Dietary fibre is that fraction of the edible part of plants or their extracts, or synthetic analogues, that are resistant to digestion and absorption in the human small intestine, usually with complete or partial fermentation in the large intestine. Dietary fibre promotes one or more of these beneficial physiological effects: laxation, reduction in blood cholesterol, and/or modulation of blood glucose. The term includes polysaccharides, oligosaccharides (degrees of polymerization >2), and lignin.
Epidemiological studies have repeatedly identified that high-fibre diets are associated with lower body weight(Reference Newby, Maras and Bakun24–Reference Du, van der and Boshuizen27). This inverse relationship can also be observed for weight gain(Reference Koh-Banerjee, Franz and Sampson93), visceral adiposity(Reference Davis, Alexander and Ventura94), cardiometabolic diseases(Reference Ludwig, Pereira and Kroenke26), type 2 diabetes(95) and colorectal cancer(Reference Murphy, Norat and Ferrari96). Clinical trials with animals and human subjects have investigated the causal role of fibre in these effects. Animal studies find supplementation with dietary fibre reduces energy intake and protects against weight gain(Reference Arora, Loo and Anastasovska97–Reference Delzenne, Cani and Daubioul100). A meta-analysis of twelve randomised controlled trials identified a reduction in body weight of obese/overweight human subjects when their diets were supplemented with soluble fibre(Reference Thompson, Hannon and An101).
Dietary fibre has been proposed to exert its benefits on host metabolism partly through its interaction with the gut microbiota. As previously stated, dietary fibres resist digestion in the human gut and reach the distal gut. The number of bacteria increases along the gut, with colon having the highest numbers. Thus in the distal gut, bacteria is at a capacity to see marked bacterial fermentation of dietary fibre. This process yields energy for bacterial growth along with gases and the side products called SCFA. The most abundant SCFA are acetate (C2), propionate (C3) and butyrate (C4), which are present in the colon in the approximate ratio of 3:1:1 although this ratio depends on the amount and type of dietary fibre and the composition of gut microbiota(Reference Cummings, Pomare and Branch102). While SCFA are waste by-products for the microbiota, for the host, their production represents the extraction of energy from the undigested material that would otherwise be wasted in the stool. Species that consume plant-rich, high-fibre diets such as gorillas (75–80 g/d) rely on this system as their main energy source(Reference Popovich, Jenkins and Kendall103). In the western world human subjects, SCFA are estimated to contribute 2–10 % of daily energy intake(Reference Bergman104). In the large intestine, most of the SCFA (mainly butyrate) are rapidly absorbed and used as an energy substrate by the colonocytes. The remaining SCFA reach the liver via hepatic portal vein where they are used as substrates for gluconeogenesis or metabolised through other pathways(Reference den Besten, Lange and Havinga105). Only a small proportion of SCFA enter the peripheral circulation. A study quantified systemic availability of colonic acetate, propionate and butyrate as 36, 9 and 2 %, respectively(Reference Boets, Gomand and Deroover106).
SCFA and appetite regulation
In the human body, SCFA are more than merely a source of energy. SCFA have been shown to act as signalling molecules through their interactions with the NEFA receptors 2 and 3 (FFAR2 and FFAR3). Acetate and propionate activate FFAR2, whereas FFAR3 can also be activated by butyrate(Reference Brown, Goldsworthy and Barnes107–Reference Nilsson, Kotarsky and Owman109). FFAR are expressed in key areas involved in the regulation of energy metabolism, including the gut, adipose tissue and skeletal muscle(Reference Brown, Goldsworthy and Barnes107, Reference Alia, Aaron and Gary110, Reference Li, Su and Zhou111).
In the gut, the FFAR are expressed in GLP-1 and PYY secreting L-cells. This discovery led to the suggestion that SCFA could stimulate the release of appetite-suppressing hormones which could explain the mechanism by which fibre influences body weight. Indeed, studies using rodent and human cell lines confirmed SCFA acted on FFAR on L-cells to stimulate the release of GLP-1 and PYY(Reference Chambers, Viardot and Psichas30, Reference Psichas, Sleeth and Murphy112, Reference Karaki, Mitsui and Hayashi113). This finding was further strengthened by FFAR2 and/or FFAR3 knock-out rodent models which showed attenuated GLP-1 and PYY secretions in response to intra-colonic propionate infusions(Reference Psichas, Sleeth and Murphy112, Reference Tolhurst, Heffron and Lam114, Reference Lin, Frassetto and Kowalik115). Dietary supplementation with fermentable carbohydrates or SCFA increased the circulating concentrations of PYY and GLP-1 in animal models and activated neurones in hypothalamic regions involved in appetite regulation(Reference Cani, Neyrinck and Maton98, Reference Tolhurst, Heffron and Lam114, Reference Anastasovska, Arora and Sanchez Canon116–Reference Reimer, Maurer and Eller118).
SCFA and energy intake
Despite the well-established link between SCFA and anorectic gut hormone release, the effect of SCFA on energy intake has been inconsistent. Several studies investigated the effect of supplementing rodent diets with SCFA on energy intake and found no effect(Reference den Besten, Bleeker and Gerding119–Reference Henagan, Stefanska and Fang122). One study supplemented high-fat diets with acetate, butyrate or propionate and showed a significant reduction in energy intake of mice following butyrate and propionate supplementations (22 and 9 %, respectively)(Reference Lin, Frassetto and Kowalik115). However, studies using oral supplementation of SCFA should be interpreted with care as the bitter taste of these supplements may cause food aversion. In another study, intragastric gavage of butyrate was found to significantly reduce food intake of mice(Reference Li, Yi and Katiraei123). Frost et al. also demonstrated that intraperitoneal administration of acetate acutely reduced food intake for 2 h although colonic administrations showed no effect(Reference Frost, Sleeth and Sahuri-Arisoylu124).
Oral SCFA failed to show an effect on food intake in human subjects(Reference Darzi, Frost and Robertson125). This may be due to SCFA being quickly absorbed in the upper gut, and thus not reaching the distal gut where they are proposed to interact with the L-cells to drive anorectic hormone release. Our research group has used inulin-propionate ester for the targeted delivery of propionate to the distal gut. In a randomised controlled study, 7 d supplementation with the inulin-propionate resulted in a reduction in ad libitum energy intake(Reference Polyviou, MacDougall and Chambers126). In another study, consumption of 10 g inulin-propionate ester reduced ad libitum food intake and increased plasma GLP-1 and PYY concentrations compared to an inulin control(Reference Chambers, Viardot and Psichas30). These results suggest that not oral but targeted colonic administration of SCFA may reduce food consumption in human subjects.
SCFA and body weight
Animal studies indicated a beneficial effect of SCFA on body weight. Studies with rodents highlighted that colonic infusions, intragastric gavage and oral supplementation of SCFA attenuated high-fat diet-induced weight gain(Reference den Besten, Lange and Havinga105, Reference Gao, Yin and Zhang120–Reference Li, Yi and Katiraei123, Reference Zhou, Pan and Xin127–Reference Hong, Jia and Pan129). Lu et al. supplemented high-fat diets with acetate, propionate, butyrate or a mixture of all three SCFA, and fed these to mice for 16 weeks. All SCFA significantly attenuated high-fat diet-induced weight gain, with acetate having the largest effect (72 % less weight gain)(Reference Lu, Fan and Li128). In one study, germ-free mice received faecal transplants from obese and lean human subjects which resulted in the development of a similar phenotype in the recipient mice(Reference Ridaura, Faith and Rey130). The lean mice had significantly higher caecal levels of propionate and butyrate, suggesting a potential benefit of SCFA in mediating the observed difference in body weight(Reference Ridaura, Faith and Rey130). Similar results were observed following faecal transplantation between mice that undergone Roux-en-Y gastric bypass and germ-free mice(Reference Liou, Paziuk and Luevano131). However, other studies have found weight gain following acetate supplementation(Reference Perry, Peng and Barry132, Reference Sahuri-Arisoylu, Brody and Parkinson133). In addition, studies using FFAR2/3 knock out animal models reported inconsistent outcomes on body weight(Reference Lin, Frassetto and Kowalik115, Reference Samuel, Shaito and Motoike134).
Only one human study has investigated the link with SCFA and body weight. Long-term (24 weeks) supplementation of inulin-propionate ester was shown to significantly reduce body weight gain in overweight participants compared to the inulin control as part of a habitual diet(Reference Chambers, Viardot and Psichas30). Only 4 % of participants in the inulin-propionate group gained significant weight (>3 % body weight) compared to 25 % in the control group.
Overall, these data highlight that SCFA stimulate the release of appetite-suppressing hormones. In human subjects, increasing colonic levels of SCFA may reduce food intake and protect against weight gain. Manipulating the colonic levels of SCFA is also possible through dietary modification since increasing fibre consumption has been shown to increase SCFA production. However, in addition to the fibre content, gut microbial composition can have a strong impact on SCFA production.
Gut microbiota composition and its effect on fermentation
Gut microbiota is a combined community of several types of microorganisms, including viruses, yeast and bacteria, with the latter being the most heavily researched and the most abundant(Reference Rinninella, Raoul and Cintoni135). This is why the gut microbiota is sometimes referred to as the gut bacteria. The human gut microbiota is dominated by six bacterial phyla: Bacteroidetes, Firmicutes, Proteobacteria, Verrucomicrobia, Fusobacteria and Actinobacteria, with the Bacteroidetes and Firmicutes making up about 90 % of the whole community(Reference Arumugam, Raes and Pelletier136, Reference Qin, Li and Raes137). Although the complete repertoire of the gut microbiota remains unrevealed, more than 500 species are estimated to reside in the GI tract(Reference Almeida, Mitchell and Boland138). The composition of the gut microbiota dictates its overall metabolism and functional capabilities including SCFA production. Indeed, the gut microbial composition has been shown to lead variations in SCFA productions(Reference Rahat-Rozenbloom, Fernandes and Gloor21, Reference Aguirre, Bussolo de Souza and Venema139–Reference Venkataraman, Sieber and Schmidt142). However, it should be noted that this area of research mainly relies on in vitro models or stool SCFA measurements due to a lack of studies directly measuring luminal SCFA production in healthy human subjects.
Many bacterial species are capable of producing acetate whereas propionate and butyrate productions are more conserved and substrate-dependent. Butyrate producers are mainly from the Firmicutes phylum(Reference Qin, Li and Raes137, Reference Barcenilla, Pryde and Martin143, Reference Louis, Young and Holtrop144). Faecalibacterium prausnitzii, Eubacterium rectale and Eubacterium hallii are primary butyrate-producing species in the human gut(Reference Barcenilla, Pryde and Martin143, Reference Louis, Young and Holtrop144). Resistant starch fermentation is believed to contribute to butyrate production by generating intermediate products that are fermentable by butyrate producers. Ruminococcus bromii (Firmicutes) and Bifidobacterium (Actinobacteria) are regarded as the main resistant starch-degrading bacteria(Reference Rossi, Corradini and Amaretti145–Reference Belenguer, Duncan and Calder147). Individuals with higher R. bromii abundance have been shown to produce more total SCFA and butyrate(Reference Baxter, Schmidt and Venkataraman140, Reference Venkataraman, Sieber and Schmidt142, Reference Ze, Duncan and Louis146). Similarly, it has been shown that individuals with a higher Firmicutes abundance have a greater SCFA production capacity marked by increased acetate and butyrate productions(Reference Rahat-Rozenbloom, Fernandes and Gloor21, Reference Aguirre, Bussolo de Souza and Venema139, Reference Venkataraman, Sieber and Schmidt142, Reference Turnbaugh, Ley and Mahowald148).
Although shared by a number of phyla, Bacteroidetes dominates the propionate producers(Reference Qin, Li and Raes137, Reference Louis, Young and Holtrop144, Reference Reichardt, Duncan and Young149). In line with this, one study showed a positive correlation between faecal propionate concentrations and the abundance of Bacteroidetes (Reference Salonen, Lahti and Salojärvi141). Other species such as Akkermansia muciniphila and the phylum Firmicutes (mainly class Negativicutes) have also been associated with propionate production(Reference Reichardt, Duncan and Young149, Reference Derrien, Vaughan and Plugge150). In particular, A. muciniphila has been identified as a key propionate producer and drew attention as a potential probiotic due to its negative correlations with diabetes and obesity(Reference Derrien, Vaughan and Plugge150–Reference Hansen, Krych and Nielsen153). Bacteroidetes mainly produce propionate from the fermentation of polysaccharides although they are also able to ferment peptides(Reference Louis, Young and Holtrop144). Accordingly, diets high in protein and lower in fibre have been associated with an increase in Bacteroidetes, reflecting a switch from carbohydrate to protein fermentation(Reference Aguirre, Eck and Koenen154, Reference David, Maurice and Carmody155).
Although relatively stable during adulthood, gut microbiota is susceptible to changes by dietary intake, which can in turn affect SCFA production. In diet switching studies, it was elegantly shown that the composition of the gut microbiota and the magnitude of bacterial fermentation can be altered using high- and low-fibre diets(Reference Robertson, Bickerton and Dennis29, Reference David, Maurice and Carmody155, Reference O'Keefe, Li and Lahti156). High-fibre diets increase SCFA production and increase gut microbiota diversity(Reference David, Maurice and Carmody155, Reference Brinkworth, Noakes and Clifton157–Reference Wu, Chen and Hoffmann161). Unsurprisingly these diets also stimulate the growth of carbohydrate fermenting bacterial species from the Firmicutes phylum(Reference David, Maurice and Carmody155, Reference Duncan, Belenguer and Holtrop158, Reference Reichardt, Vollmer and Holtrop162, Reference Walker, Ince and Duncan163). Diets high in resistant starch were found to stimulate the growth of R. bromii and Bifidobacterium (Reference Salonen, Lahti and Salojärvi141, Reference Brinkworth, Noakes and Clifton157, Reference Hald, Schioldan and Moore159, Reference Reichardt, Vollmer and Holtrop162–Reference Leitch, Walker and Duncan165). Conversely, western-style low-fibre, high-protein/fat diets lowered bacterial fermentation, reduced bacterial diversity and increased the numbers of Bacteroidetes (Reference Aguirre, Eck and Koenen154, Reference David, Maurice and Carmody155, Reference Wu, Chen and Hoffmann161, Reference Sonnenburg, Smits and Tikhonov166).
These studies highlight that diet and gut microbiota act together to impact SCFA production, and thus the subsequent effects of SCFA in the human body, including energy intake and body weight. Differences have been observed between the gut microbial composition of lean and obese subjects, highlighting the potential impact of gut microbiota on body weight(Reference Ley, Backhed and Turnbaugh167–Reference Schwiertz, Taras and Schäfer169). In addition, bariatric surgery has been shown to modulate gut microbial composition which is proposed to contribute to the weight loss following surgery(Reference Zhang, DiBaise and Zuccolo170, Reference Furet, Kong and Tap171). However, this topic is beyond the scope of the present paper and reviewed in detail elsewhere(Reference Castaner, Goday and Park172, Reference Ulker and Yildiran173).
Food structure
Nutritional research has largely considered the effect of food and diets on human health based on the chemical compositions of foods (i.e. macronutrients and energy). However, this approach alone is insufficient as demonstrated by the variability in metabolic response to foods with the same energy and macronutrient profiles. Beyond macronutrient composition, food structure is fundamental for dictating the food behaviour, and thus how it is digested and processed within the GI tract. This in turn can impact on postprandial response and metabolism, such as microbial fermentation and appetite control.
Food structure and digestion
Food structure relates to the assembly of molecules making up food which can be a result of natural formation, domestic processing (cooking, blending, etc.), industrial processing or a combination. There are different levels of food structures which can impact digestion and subsequent postprandial metabolism. As previously mentioned, the main substrate for microbial fermentation is carbohydrate. Therefore, this review will focus on carbohydrate structures.
Molecular level: starch
Starch is a glucose polymer, and the main form of carbohydrate storage within plants. The glucose units can be linked with α-1,4 glycosidic bonds to form straight helical chains called amylose(Reference Berg, Tymoczko and Stryer174). Alternatively, the glucose units can be linked with a combination of α-1,4 and α-1,6 glycosidic bonds to form branched polymers called amylopectin. The proportion of these starch configurations differs between different plants and starch types(Reference Fishman, Cooke and White175). In general, starchy foods such as barley, rice, wheat would contain 20–30 % amylose and 70–80 % amylopectin(Reference Svihus, Uhlen and Harstad176). Amylose is a straight chain, and thus with less surface area available for enzymatic actions, its digestion is slower compared to the highly branched amylopectin(Reference Lovegrove, Edwards and De Noni177). In vitro digestion studies have demonstrated this eloquently, for example, the digestion rate of different rice grains being shown to increase with the increasing ratio of amylose to amylopectin(Reference Syahariza, Sar and Hasjim178). In clinical human studies, high amylose starch supplementation resulted in attenuated postprandial glucose and insulin responses compared to high amylopectin starch, indicative of slower digestion from the former(Reference Behall and Howe179, Reference Behall, Scholfield and Yuhaniak180).
At greater length scale, amylose and amylopectin chains are further assembled into granules consisting of a ratio of highly organised, dense pseudo-crystalline regions and less organised amorphous structures(Reference Mishra, Hardacre and Monro181). The pseudo-crystalline regions are abundant in raw vegetables such as potatoes and unripe bananas and they are more resistant to digestion by α-amylase resulting in slower and sometimes incomplete digestion. In a study that investigated the in vitro digestion of potato, pea, maize, rice, barley and wheat starches, the digestion rate was found to decrease with the increasing amount of crystalline structures(Reference Martens, Gerrits and Bruininx182).
Food cellular structure
At its most basic, food cellular structures include animal, fungal and plant cells. Unlike animal cells, plant cells have cell walls providing structural support and shielding intracellular nutrients(Reference Brett and Waldron183). These cell walls are made up of indigestible carbohydrates (i.e. fibres) such as cellulose, hemicellulose, pectin and non-carbohydrates such as lignin, proteins and water(Reference Burton, Gidley and Fincher184). The relative proportion of these building blocks differs based on the type, function and maturity of plant tissue, which in turn dictates the permeability and strength of the cell wall and the digestive fate of these foods(Reference Padayachee, Day and Howell185). For example, high amounts of cell wall lignin relate to the string-like structure of asparagus reducing the permeability of cell wall and thus hindering digestive enzyme access and nutrient bioaccessibility(Reference Harris and Smith186).
Bioaccessibility is defined as the proportion of consumed nutrients available for absorption in the human gut. Food cellular structures have been shown to impact bioaccessibility(Reference Parada and Aguilera187). Digestive enzymes need direct contact with the nutrients inside the cellular structures to be able to digest them. Endogenous enzymes in the human body are unable to digest plant cell walls (i.e. fibre), and therefore enzymes can only act to break down macronutrients within plant cells if they diffuse through the cell walls or if the cell walls are ruptured as part of the digestion process. As a result, plants with strong and less permeable cell walls may undergo more attenuated and incomplete digestion within the GI tract which results in a slower and lower nutrient release. In vitro digestion studies have shown slower hydrolysis of cellular starch compared to extracellular starch(Reference Edwards, Grundy and Grassby31), and in applied food examples, kidney bean and chickpea cells, which have remained intact following cooking, have resulted in a slower and incomplete in vitro digestion(Reference Mishra, Hardacre and Monro181). In vivo, human studies demonstrated an attenuated postprandial glycaemia and lipidaemia following the consumption of foods with intact cellular structures compared to macronutrient and energy-matched foods with disrupted structures, indicating a slower nutrient release from the former(Reference Edwards, Grundy and Grassby31, Reference Berry, Tydeman and Lewis188–Reference Jenkins, Wesson and Wolever190). In some cases, intact cells may protect cellular starch from digestion both of which reach the distal gut. Ileal samples from healthy human subjects were found to contain intact cells with cellular starch molecules, indicating that these cells escaped digestion in the upper gut(Reference Noah, Guillon and Bouchet191). Food cellular structures are further organised into tissues at greater length scales, which further dictates their digestive behaviour.
Mechanical processing in human body
During digestion, food structures are altered due to mechanical stress, actions of digestive enzymes and physicochemical conditions of the GI tract such as pH(Reference Grundy, Edwards and Mackie192). Oral processing (chewing) is the first stage of digestion and reduces the particle size of food, thus increasing the surface area for enzymatic digestion. This process changes the food matrices by separating tissues, and on a cellular level separating and/or rupturing cell wall surfaces. Depending on cell wall structure and conformation, such structures have different strengths of intracellular adherence and tendencies to rupture or separate under mechanical stress(Reference Swackhamer and Bornhorst193). For example, high pectin levels in the cell walls usually indicate greater potential for cell separation. Under digestion, the cells of nuts, raw hard vegetables and seeds have a greater tendency to rupture, whereas cells of cooked foods such as legumes tend to separate and remain intact(Reference Grundy, Grassby and Mandalari194, Reference Grundy, Carrière and Mackie195). This means that most legume cells remain intact following chewing, the result of which can provide one possible mechanism to explain their attenuated postprandial glycaemic response, due to lower enzymatic exposure to intracellular starch. Conversely, ruptured cell walls provide greater enzymatic exposure to intracellular starch (Fig. 2). Studies where foods have been swallowed without oral processing have demonstrated such, through reduced postprandial glycaemia, indicating a slower nutrient release due to the protection of cell walls(Reference Read, Welch and Austen196).
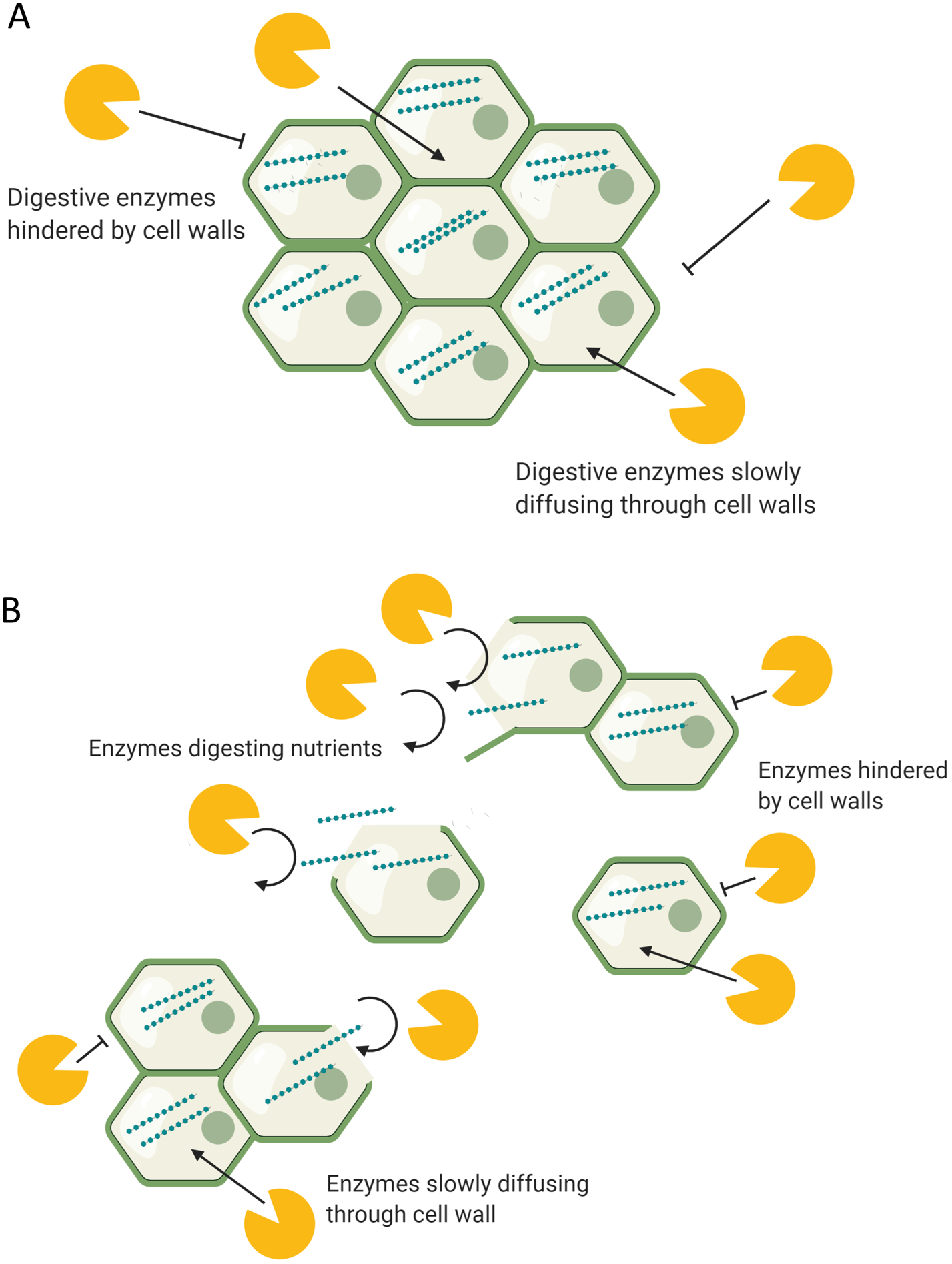
Fig. 2. (Colour online) The digestion of (a) intact and (b) disrupted food structures. (a) Digestive enzymes in the body have limited access to the intracellular nutrients, hindered by intact food structures and cell walls. (b) Digestive enzymes have access to acellular nutrients released from ruptured/disrupted cellular structures. Separated cells remain intact and limit enzyme access to intracellular nutrients.
Mechanical digestion continues in the stomach and small intestine where the digesta is constantly mixed by peristaltic gut movements. Plant foods with strong food structures can endure the contractions created by the GI tract and reach the distal gut relatively undigested. One study using intubation technique to collect samples from the distal ileum of healthy volunteers found ileal digesta contained intact bean cells encapsulating starch molecules, indicating that these cells were resistant to digestion in the upper GI tract(Reference Noah, Guillon and Bouchet191). Another study with healthy ileostomy patients demonstrated intact carrot cells containing intracellular nutrients in the ileal effluents(Reference Tydeman, Parker and Faulks197). Such findings, further highlighting intact cellular structures with encapsulated nutrients reaching the distal ileum, have been repeatedly confirmed within ileostomy patients(Reference Edwards, Grundy and Grassby31, Reference Mandalari, Faulks and Rich198–Reference Birkett, Mathers and Jones200). Theoretically, the greater the protective effect of food structure, the greater is the amount of fermentable substrates delivered to the distal gut for fermentation. This may be indicating the beneficial role of food structure in stimulating appetite suppression through the proposed mechanisms within this review.
Industrial and domestic processing
Food processing has been shown to change food structures, typically leading to more digestible products. Thermal treatment in the presence of water (boiling) has been shown to cause gelatinisation of starch(Reference Olkku and Rha201). Gelatinisation results in the loss of intermolecular bonds and pseudo crystalline structures, resulting in a more digestible compound. For example, raw potato starch or raw oats eaten as muesli are almost completely indigestible to human subjects. Upon cooking, the starch in these foods gelatinises and can be easily digested, marked by a higher postprandial glycaemia(Reference Mishra, Hardacre and Monro181). However, if the gelatinised starch is left to cool down, the dissociated starch molecules randomly re-crystallise in a disorganised manner (retrograding). This generates compounds more resistant to digestion (resistant starch). In a study with healthy ileostomy patients, cooked potatoes resulted in 3 % starch losses in the ileal effluents whereas cooked and cooled potatoes resulted in 12 %(Reference Englyst and Cummings202).
Beyond starch structures, processing has been shown to alter cell walls, typically leading to more permeable, weak or ruptured structures(Reference Edwards, Grundy and Grassby31–Reference Grassby, Mandalari and Grundy37). For example, the fine milling process has been shown to rupture cell walls(Reference Edwards, Grundy and Grassby31, Reference Moelants, Cardinaels and Van Buggenhout203). Others such as homogenisation, canning and cooking were shown to denature cell walls leading to rupture or weakening(Reference Padayachee, Day and Howell185). As previously explained, without the hindrance of rigid, impermeable cell walls, digestive enzymes can easily access the intracellular nutrients and hydrolyse them into absorbable molecules. Weak cell walls may also be more susceptible to rupture under mechanical digestion in the GI tract. This can lead to more digestible and bioaccessible food products(Reference Parada and Aguilera187). Singh and coworkers reviewed the effect of different types of food processing on starch digestibility and found an increase in digestibility with processing(Reference Lin, Frassetto and Kowalik115). In another study, in vitro digestion of whole and finely milled almonds resulted in higher energy extraction from the milled almonds(Reference Mandalari, Faulks and Rich198). This was supported by microscopic analysis of almonds finding ruptured almond cells following the milling process while the whole almonds contained intact cells with encapsulated nutrients. Another in vitro digestion study estimated that only 5⋅5 % of lipids were released from whole almonds compared to 42 % from almond starch(Reference Grassby, Picout and Mandalari204). These results were repeated in a healthy ileostomy patient who digested 96⋅5 % of lipids from milled almonds compared to only 56⋅5 % from whole almonds(Reference Grassby, Mandalari and Grundy37). Increased nutrient availability of processed foods has been demonstrated by other studies using cooked, pasteurised and milled products(Reference Edwards, Grundy and Grassby31–Reference Langkilde, Champ and Andersson36).
More efficient digestion and absorption in the upper gut translates into lower levels of nutrients reaching the distal gut. In a study with healthy ileostomy patients, Livesey et al. demonstrated that the starch losses in the ileal effluents decreased 3-fold following the consumption of finely milled barley compared to coarse barley(Reference Livesey, Wilkinson and Roe199). An analysis of the ileal effluents found that the lost nutrients were still encapsulated within the intact cellular structures. Langkilde et al. repeated this finding in an experiment where ileostomy patients were fed cooked and raw banana starch(Reference Langkilde, Champ and Andersson36). Raw banana starch consumption resulted in more than three times higher amounts of starch losses in the ileal effluents (6⋅3 (sd 0⋅4) v. 21⋅4 (sd 0⋅6)). Intact cellular structures with encapsulated nutrients were found in the ileal effluents of raw banana starch group. This indicated that the observed differences in both of these studies could be due to more nutrients being absorbed from the disrupted structures of processed foods while the intact cellular structures protected the nutrients in the raw diets.
Two studies showed contradictory results. Edwards et al. fed coarse and finely milled porridge to healthy ileostomy patients and found no difference in the starch losses in the ileal effluents(Reference Edwards, Grundy and Grassby31). This was explained by the finding of intact but empty cellular structures in the effluents following the consumption of coarse oats. Combined with the in vitro findings, this indicated that the cell walls were permeable to digestive enzymes although they were resistant to digestion. Another study found no difference between the ileal effluents following cooked and raw carrot consumption(Reference Tydeman, Parker and Faulks197). This was explained by the failure of cooking to disrupt cellular structures and highlighted that not all food processing results in the disruption of cellular structures.
Food structure, microbial fermentation and appetite control
It is well established that food structures dictate the digestive fate of consumed nutrients(Reference Grundy, Edwards and Mackie192). It is therefore unsurprising that food structures can also influence appetite control. Foods with rigid structures may require a longer chewing time while others may be consumed rapidly. Accordingly, diets high in plant matter (fibre) have been shown to reduce eating rate(Reference Louis, Young and Holtrop144). A slow eating rate prolongs the oral exposure of food which transmits orosensory satiety signals to the brain, resulting in a prolonged appetite suppression(Reference Rossi, Corradini and Amaretti145). The importance of this is demonstrated by the attenuated appetite suppression observed following direct infusion of foods into the stomach or duodenum(Reference Ze, Duncan and Louis146, Reference Belenguer, Duncan and Calder147). Li et al. also demonstrated that increasing oral exposure of food (15 v. 40 chews) resulted in higher postprandial GLP-1 concentrations and reduced ad libitum energy intake in obese and lean subjects(Reference Turnbaugh, Ley and Mahowald148). In another study, eating at a slower rate increased the postprandial GLP-1 and PYY levels(Reference Reichardt, Duncan and Young149). Food structure was also found to impact gastric emptying which contributes to appetite suppression. In a randomised cross-over study, Mackie and coworkers demonstrated slower gastric emptying following the consumption of semi-solid meals compared to liquid(Reference Derrien, Vaughan and Plugge150).
As previously described, food structures can alter the amount of nutrients reaching the distal gut which can impact bacterial fermentation (Fig. 3). While cell walls are indigestible by human enzymes, they are susceptible to bacterial enzymes and fermentation, producing SCFA(Reference Reichardt, Vollmer and Holtrop162). The composition of the plant cell wall is a major determinant of bacterial fermentation since different fibres have different fermentation capacities. Complex, insoluble fibres such as cellulose and hemicellulose are fermented slowly and poorly by the gut bacteria whereas soluble fibres such as pectin and β-glucan are highly fermentable(Reference Reichardt, Vollmer and Holtrop162, Reference Holscher205). Bacterial degradation of plant cell walls may also expose the intracellular starch that also acts as an efficient substrate for bacterial fermentation. However, as previously mentioned, gut microbiota composition is also a determinant of SCFA production. The presence of certain microbial groups may enhance the fermentation of complex structures such as resistant starches(Reference Rossi, Corradini and Amaretti145–Reference Belenguer, Duncan and Calder147).
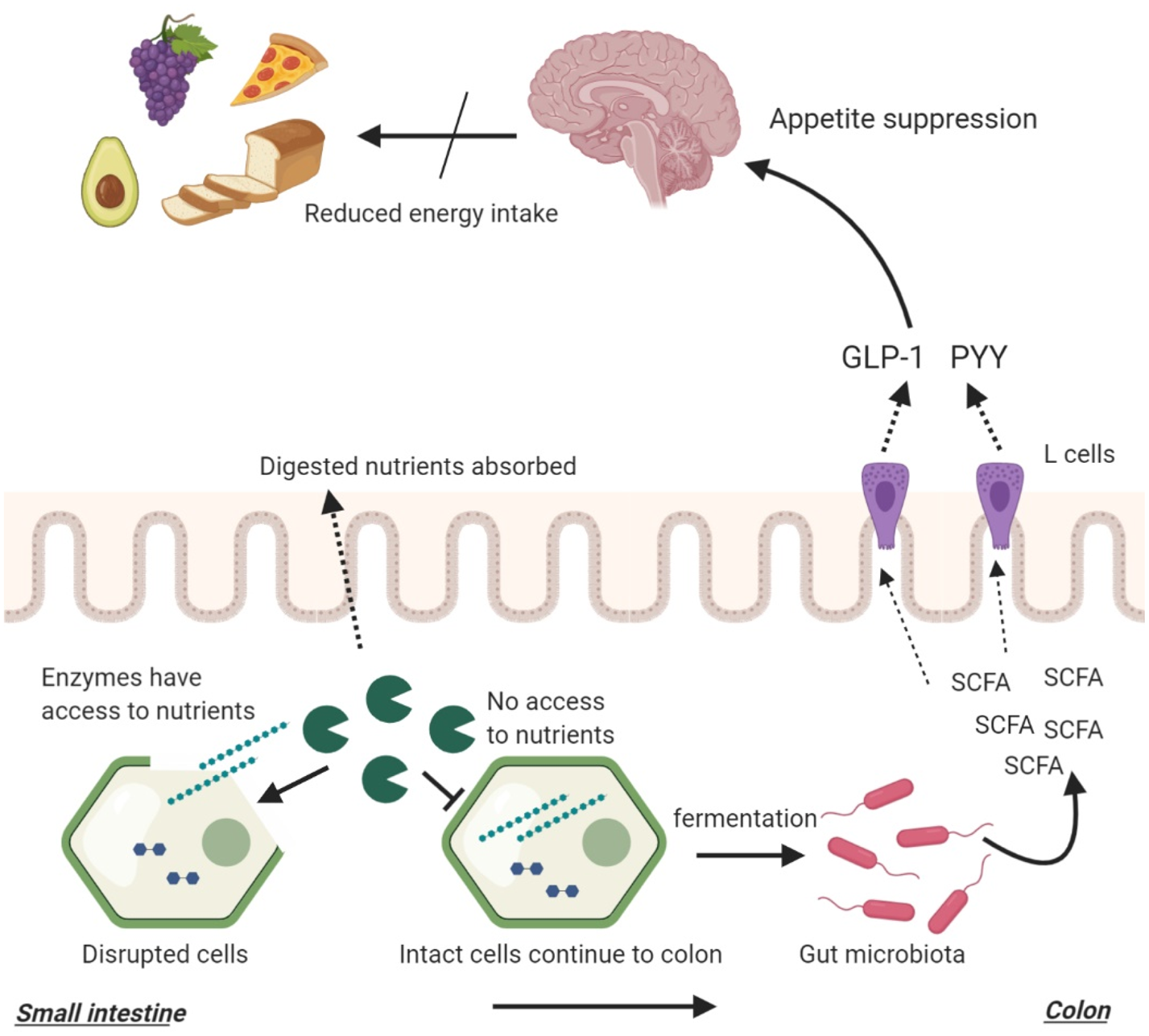
Fig. 3. The interplay between food structures, digestion, microbial fermentation and appetite regulation. Intact cellular structures arrive large intestine where they become available for bacterial fermentation. This process yields SCFA that stimulate the release of peptide YY (PYY) and glucagon-like peptide 1 (GLP-1) from intestinal L-cells. PYY and GLP-1 signal to the brain to reduce appetite.
In one study, ileal effluents with higher fibre and starch losses were found to result in higher in vitro SCFA production(Reference Silvester, Englyst and Cummings206). This study highlights that the amount of nutrients reaching the distal gut is a determinant of microbial fermentation. In a previously mentioned study, ileal effluents were collected from healthy ileostomy patients following the consumption of raw or cooked banana starch(Reference Langkilde, Champ and Andersson36). The in vitro fermentation of the effluents resulted in significantly higher SCFA production following the inoculation of raw banana effluents which contained higher amounts of fibre and starch. In another study, the effect of food processing was investigated using native and finely milled whole grains(Reference Hernot, Boileau and Bauer207). It was found that processing reduced the resistant starch components of foods which in turn reduced their in vitro SCFA production. These studies highlighted that macronutrient, fibre and energy-matched foods can lead to different microbial fermentation due to a difference in food structure brought by food processing with more ‘resistant’ food structures resulting in greater SCFA production (Fig. 3). In addition, it highlighted that cooking and milling (processing) can reduce the amount of fibre/starch reaching the distal gut and reduce bacterial fermentation through increasing digestibility. This can in return reduce appetite suppression.
These findings indicate that generally, processed foods could be less satiating compared to raw, unprocessed alternatives. Evidence from cross-sectional studies suggests that high consumption of highly processed foods is associated with excess body weight(Reference Juul, Martinez-Steele and Parekh208–Reference Canella, Levy and Martins213). One study found an inverse correlation between the degree of processing and the satiety index of ninety-eight commonly consumed foods(Reference Fardet214). Another study used subjective measures of appetite and demonstrated higher satiety following the consumption of raw carrots compared to cooked carrots(Reference Gustafsson, Asp and Hagander215). In a randomised cross-over trial, Mori et al. demonstrated that whole almonds reduced ad libitum food intake in healthy volunteers more than energy, macronutrient and fibre-matched almond butter(Reference Mori, Considine and Mattes216). The authors commented that this difference may be due to increased orosensory time and reduced gastric emptying. In another study, it was shown that supplementation with native banana starch, which has been previously shown to carry intact cellular structures to the distal gut(Reference Langkilde, Champ and Andersson36), reduced ad libitum food intake compared to supplementation with available banana starch indicative of appetite suppression(Reference Ble-Castillo, Juarez-Rojop and Tovilla-Zarate217). In a recently published randomised cross-over study, Hall et al. fed participants energy, macronutrient and fibre-matched ultra-processed (as per NOVA classification) and unprocessed diets for 14 d(Reference Hall, Ayuketah and Brychta218). Ad libitum energy intake was found to be about 2092 kJ (500 kcal)/d greater in the ultra-processed diet group. This translated into participants' body weight and fat mass increase in the ultra-processed diet group. Fasting PYY levels were also found to be significantly higher in the unprocessed diet group. Furthermore, the eating rate was also significantly lower in this group.
However, evidence in this area remains inconclusive and limited. There are still no studies investigating the causal link between food structures, bacterial fermentation and appetite suppression directly, with current hypothesis being based on the mechanistic link proposed in this review.
Conclusion
Current evidence highlights the beneficial effects of dietary fibre intake on energy metabolism. This benefit is partly mediated by the interaction of fibre with the gut microbiota which produces SCFA. These bacterial metabolites have been shown to stimulate the release of appetite-suppressing hormones GLP-1 and PYY. The limited evidence in human subjects highlights that increasing colonic levels of SCFA may reduce food intake and protect against weight gain. Processing can alter food structures, typically reducing the amount of fibre reaching the distal gut and thus reducing substrates for bacterial fermentation and its subsequent benefits on the host metabolism including appetite suppression. Observational studies show an association between high processed food consumption and higher body weight. One randomised controlled trial in human subjects demonstrated a higher ad libitum energy intake from processed foods compared to unprocessed foods which resulted in weight gain over 2 weeks. However, scientific evidence linking food structure, bacterial fermentation and appetite control is scarce. The small amount of available evidence is mostly dependent on ileostomy studies which do not reflect the conditions of an intact gut. More studies in healthy human subjects are needed to grow understanding in this area.
Acknowledgement
I would like to acknowledge the support and help of my colleagues and supervisors at Imperial College London for me to compete at the FENS postgraduate competition and write this review article.
Financial Support
This article presents a review on a topic that is researched by our team. This work is funded by UK Biotechnology & Biological Sciences Research Council (BBSRC) (BB/N016847/1) and Nestec Ltd and supported by the NIHR CRF and BRC at Imperial College Healthcare NHS Trust. The views expressed are those of the authors and not necessarily those of our funders, the NHS, the NIHR or the Department of Health. The Section of Endocrinology and Investigative Medicine is funded by grants from the MRC, BBSRC, NIHR, an Integrative Mammalian Biology (IMB) Capacity Building Award, an FP7-HEALTH-2009-241592 EuroCHIP grant and is supported by the NIHR Biomedical Research Centre Funding Scheme. G. F. holds an NIHR Senior Investigator Award.
Conflict of Interest
None.
Authorship
The authors had joint responsibility for all aspects of preparation of this paper.






