- EAA
essential amino acids
- MPS
muscle protein synthesis
Human skeletal muscle protein turnover comprises the processes of both muscle protein synthesis (MPS) and muscle protein breakdown. These two processes are ongoing and simultaneous and provide for a mechanism to trim protein components and modify protein composition within muscle fibres. In fact, remodelling of the muscle proteome is the underlying mechanism of skeletal muscle plasticity in response to different loading patterns such as would occur during weightlifting, which leads to hypertrophy(Reference Burd, Tang and Moore1–Reference Rennie, Wackerhage and Spangenburg3). Thus, imbalances between MPS and muscle protein breakdown in adults dictate a net gain (i.e. hypertrophy) or loss (i.e. atrophy) of muscle fibre protein. For most adults in their third, fourth and even their fifth decade of life their muscle mass is constant and thus MPS= muscle protein breakdown. However, beyond the fifth decade of life, sarcopenic muscle loss begins to occur and muscle mass slowly declines(Reference Evans4, Reference Paddon-Jones and Rasmussen5). What has been repeatedly shown, however, is that resistance exercise can promote increases in MPS(Reference Biolo, Maggi and Williams6–Reference Phillips, Tipton and Ferrando8). A number of reviews have pointed to the fact that repeated elevations in MPS create periods of positive net protein balance that sum up to create hypertrophy(Reference Phillips2, Reference Rennie, Wackerhage and Spangenburg3). The main questions examined in this review are how the timing of protein consumption in concert with exercise affects rises in MPS, how the source of dietary protein ingested can affect rises in MPS and ultimately hypertrophy.
Protein enhances exercise-induced rises in muscle protein synthesis: timing effects
When protein is consumed after resistance exercise, the effects of resistance and the accompanying hyperaminoacidemia on MPS are synergistic and an even greater stimulation of MPS occurs(Reference Moore, Tang and Burd9–Reference Biolo, Tipton and Klein11). The resultant net accumulation of muscle protein is thought to sum over time to yield muscle hypertrophy(Reference Phillips2, Reference Rennie, Wackerhage and Spangenburg3). Empirical support for this thesis has been observed. For example, Wilkinson et al.(Reference Wilkinson, Tarnopolsky and MacDonald12) showed a greater acute post-exercise accumulation of muscle protein with milk v. matched soya protein consumption. This acute finding when practised chronically during a 12-week training study resulted in net greater gains in muscle fibre hypertrophy and whole-body lean mass(Reference Hartman, Tang and Wilkinson13). In the opposite direction, that is, induction of muscle atrophy, declines in acute fasted-(Reference de Boer, Maganaris and Seynnes14, Reference Glover, Phillips and Oates15) and fed-state(Reference Glover, Phillips and Oates15) rates of MPS were quantitatively predictive of changes in muscle cross-sectional area(Reference Phillips, Glover and Rennie16). Thus, acute changes in MPS, but not changes in muscle protein breakdown which are 3–5 times less than MPS during a given day, are qualitatively predictive of long-term phenotypic changes.
The studies of post-exercise protein consumption are unequivocal in their finding of an enhanced rate of MPS after exercise(Reference Biolo, Maggi and Williams6, Reference Moore, Tang and Burd9, Reference Moore, Phillips and Babraj10, Reference Tipton, Ferrando and Phillips17). In contrast, only a few studies of pre-exercise protein(Reference Tipton, Elliott and Cree18, Reference Fujita, Dreyer and Drummond19) and amino acid(Reference Tipton, Rasmussen and Miller20) consumption have been conducted with equivocal results in terms of benefits of pre-exercise feeding on MPS. This is perhaps not overly surprising, since it appears that during exercise a low cellular energy charge(Reference Bolster, Crozier and Kimball21) or increased Ca concentration(Reference Rose, Alsted and Jensen22) appear to suppress protein synthesis. Thus, the provision of protein prior to exercise would result in a hyperaminoacidemia during exercise when the muscle would be unable to mount an anabolic response. Provision of protein during a workout would in theory be no more beneficial than pre-workout protein consumption since amino acids would, depending on the length of the workout, be appearing in the circulation when muscle is not able to make good use of them. One study has examined peri-exercise protein consumption and the subsequent response of MPS(Reference Beelen, Koopman and Gijsen23) and has shown benefits in terms of elevating post-exercise MPS. This was a rather prolonged (2 h) exercise bout and it was impossible to tell from the timing of biopsies whether the rise in MPS actually occurred during the bout or whether it occurred sometime afterward and may in fact have occurred during both periods. Nonetheless, consumption of protein and carbohydrate during the exercise bout did result in a 50% greater rise in MPS post-exercise implying that some benefit may be gained by peri-workout protein consumption.
A consistent finding has been that post-workout protein consumption promotes increments and MPS(Reference Moore, Tang and Burd9, Reference Biolo, Tipton and Klein11, Reference Wilkinson, Tarnopolsky and MacDonald12), which ultimately sum up to yield muscle hypertrophy. The rise in MPS occurs rapidly following a maximally stimulatory bolus dose of protein(Reference Moore, Robinson and Fry24) being apparent within the first hour after resistance exercise and peaking at 3 h post-exercise(Reference Moore, Tang and Burd9). Interestingly, the dose of protein in younger men that maximally stimulated MPS was found to be approximately 20 g(Reference Moore, Robinson and Fry24), which corresponds to approximately 8·5 g essential amino acids (EAA) or 1·5 g leucine. The EAA and leucine content of the protein are mentioned because it appears that only the EAA are required to stimulate MPS(Reference Volpi, Kobayashi and Sheffield-Moore25) and leucine is a key metabolic regulator of MPS through the activation of the mammalian target of rapamycin pathway(Reference Crozier, Kimball and Emmert26, Reference Anthony, Reiter and Anthony27). The addition of carbohydrate to this dose of protein does not appear to enhance MPS further than the protein itself (AW Staples and SM Phillips, unpublished results), consistent with the concept that insulin is a permissive hormone for protein synthesis and not stimulatory(Reference Greenhaff, Karagounis and Peirce28). Thus, a prescription for optimal stimulation of MPS post-exercise would appear to encompass the following: at least 25 g high-quality protein containing at least 8–10 g EAA, higher leucine content would appear to be advantageous, delivered as soon as possible post exercise.
Different dietary source proteins elicit different responses in muscle protein synthesis
A number of studies have now shown that different proteins elicit different anabolic responses when consumed at both a whole-body(Reference Lacroix, Bos and Leonil29–Reference Bos, Metges and Gaudichon31) and skeletal muscle(Reference Wilkinson, Tarnopolsky and MacDonald12, Reference Tang, Moore and Kujbida32) level. What is clear is that rates of digestion of proteins, in addition to the amino acid content of the protein, will dictate the amplitude and duration of the rise of EAA and leucine, which will affect the degree of stimulation of MPS. The concept of a leucine trigger or threshold for the activation of MPS has been advanced by a number of groups based on different observations(Reference Burd, Tang and Moore1, Reference Koopman, Verdijk and Manders33–Reference Norton, Layman and Bunpo35). A notable, albeit in vitro, observation is that C2C12 myotubes in culture show activation of portions of the mammalian target of rapamycin signalling pathway in response to a number of amino acids, but only leucine triggers an increase in signalling of mammalian target of rapamycin, eucaryotic initiation factor 4E binding protein and p70S6 kinase(Reference Atherton, Smith and Etheridge36). It appears that leucine has a controlling influence over the activation but not the duration of MPS(Reference Norton, Layman and Bunpo35), but differing doses of leucine elicit a graded response in MPS in terms of feeding at rest(Reference Cuthbertson, Smith and Babraj37) and post exercise(Reference Moore, Robinson and Fry24). The role of EAA therefore in the activation of MPS is questionable and one wonders whether EAA are simply substrate and not also signalling molecules. Clearly, more work remains to be performed to ascertain the interrelated nature of leucine and EAA in activating and maintaining a robust MPS response. However, for the importance of this discussion, it would seem that high-quality proteins (i.e. protein digestibility corrected amino acid score (PDCAAS) of >1·0) that have a high leucine content would be beneficial for the stimulation of MPS. One caveat to these guidelines would be that the digestion of the protein would have to be rapid providing a peak in leucine concentration to result in leucinemia of sufficient magnitude to reach the leucine threshold and activate MPS. This is likely critically important for older subjects who show a relative resistance to hyperaminoacidemia in terms of stimulation of MPS(Reference Cuthbertson, Smith and Babraj37). In older subjects, it appears that leucinemia needs to be higher to trigger rises in MPS(Reference Katsanos, Kobayashi and Sheffield-Moore34, Reference Rieu, Balage and Sornet38, Reference Guillet, Prod'homme and Balage39), which emphasises the role of higher quality, and specifically higher leucine content, proteins in the prevention of an age-related decline in muscle mass.
Work from our lab indicated that dairy proteins specifically those found in fluid milk were superior in eliciting acute rises in MPS than isonitrogenous and isoenergetic quantities of soya protein(Reference Wilkinson, Tarnopolsky and MacDonald12). The observation reported in that published paper was that total aminoacidemia was slower in milk than the soya-based drink(Reference Wilkinson, Tarnopolsky and MacDonald12). This reflects the fact that milk is by composition 80% casein and 20% whey and that casein is digested slowly, whereas whey is rapidly digested(Reference Boirie, Dangin and Gachon40). However, the appearance of leucine in the systemic circulation was markedly different and actually more rapid in the milk condition (Fig. 1). Thus, while the total aminoacidemia is slower with milk consumption the leucinemia is greater and more prolonged with milk consumption than soya presumably reflecting the contribution of the digestion of whey proteins within milk, which are higher in leucine than both casein and soya(Reference Wilkinson, Tarnopolsky and MacDonald12).
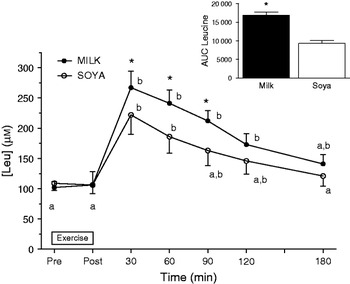
Fig. 1. Whole blood leucine concentration (μM) following resistance exercise from subjects who consumed 500 ml fluid skim (low fat) milk and an isonitrogenous and isoenergetic quantity of a soya drink (drawn with data from Wilkinson et al.(Reference Wilkinson, Tarnopolsky and MacDonald12)). AUC, area under the curve. Means with different letters are significantly different from each other (P<0·05). *Indicates a significant difference from the soya condition at the same time point or from each other in total (P<0·05). Values are means with their standard errors.
When comparing the digestion of individual proteins contained within milk, we observed that whey, as a hydrolysed protein, resulted in a pronounced and rapid hyperaminoacidemia and leucinemia compared to both isonitrogenous quantities of soya and casein. While all of these proteins have excellent PDCAAS scores (whey=1·15, casein=1·23 and soya=1·04), and are thus considered complete, the pattern of aminoacidemia and, in particular, leucinemia affected the rise in resting and post-prandial MPS. Specifically, whey and soya promoted increases in resting MPS that were greater than soya, but increments in post-resistance exercise MPS were greater in whey than both soya and casein(Reference Tang, Moore and Kujbida32). These findings are different than those seen with whole-body measurements(Reference Boirie, Dangin and Gachon40, Reference Dangin, Boirie and Garcia-Rodenas41), but it needs to be realised that only 25% of the whole-body response is due to muscle protein and acute changes in whole-body protein turnover with feeding will reflect the much more rapidly turning over proteins of the gut(Reference Nakshabendi, Obeidat and Russell42) and not those in muscle. This is an important point and one that underpins why some have concluded that whey protein cannot sustain an anabolic response compared to more slowly digested proteins(Reference Lacroix, Bos and Leonil29) (casein or milk proteins). Recently, we reviewed the evidence for the efficacy of whey as exercise supplement in supporting resistance training-induced gains in lean mass and found that milk proteins and particularly whey were more effective than carbohydrate or soya supplements(Reference Phillips, Tang and Moore43).
Conclusions
Post-exercise consumption of protein is the most effective strategy to induce increments in MPS and promote muscle mass gains. The increment in MPS is maximally stimulated at a dose of protein of approximately 25 g or 10 g EAA. This rise is based solely on protein consumption and is not augmented by carbohydrate, at least when protein is adequate. Leucine plays a key role in the stimulation of MPS and appears to be a key activator in switching on MPS, which appears important in the elderly and also following resistance exercise. Thus, high-quality, rapidly digested, leucine-rich proteins such as whey protein would appear to be ideal candidates for stimulating MPS and promotion of hypertrophy.
Acknowledgements
S. M. P. is the recipient for grants from the Canadian Natural Science and Engineering Research Council (NSERC), the US National Dairy Council and the Canadian Institutes for Health Research (CIHR) and is grateful for those agencies for funding portions of the work presented here. The author declares no conflict of interest.



