- HBE
Harris Benedict equation
- MREE
measured resting energy expenditure
- PN
parenteral nutrition
- RYGB
Roux en Y gastric bypass
Bariatric surgery, complications and nutrition support
The use of bariatric surgery to treat morbid obesity has significantly increased internationally over the past decade; for example, procedures in North America have increased from 103 000 in 2003(Reference Buchwald and Williams1) to >200 000 in 2009(2). North America currently conducts the largest number of operations. Other countries performing large numbers of this type of surgery are France, Belgium, Brazil, Italy, Australia, Egypt, Mexico and Spain(Reference Buchwald and Williams1). However, growth is not limited to these countries as the rapid expansion in bariatric surgery procedures over the past 10 years is a worldwide phenomenon. Surgery is now considered the best method of achieving long-term weight loss and decreased overall mortality in the morbid obese who have failed all other attempts to lose weight(Reference Sjostrom, Narbro and Sjostrom3, Reference Picot, Jones and Colquitt4). As well as proving to be clinically effective, the 2009 systematic review and economic evaluation for the Department of Health in England also deemed it to be a cost-effective intervention for moderate to severely obese people compared with non-surgical interventions(Reference Picot, Jones and Colquitt4). Bariatric surgery is now recommended by the National Institute for Health and Clinical Excellence(5) as a treatment option for those with BMI >35 kg/m2 with co-morbidities and it is the first line treatment in those with BMI >50 kg/m2. The 2010 statistics on obesity in England show that bariatric surgery admissions to the National Health Service are increasing annually. There were 4221 admissions to the National Health Service for bariatric surgery in 2008–2009 and this is more than double the numbers in 2006–2007(6).
With the increase in the number of surgery cases comes a significant proportion that may also experience adverse complications. The complications can be life threatening requiring admission to the intensive care unit. The management of gastrointestinal complications usually involves avoiding oral diet for a period of time and the instigation of nutrition support. Provision of nutrition support particularly when acutely unwell, can be complicated by the presence of obesity and nature of the surgery. Calculating nutritional requirements can be controversial in this group of patients. Other challenges arise, such as pre-operative micronutrient deficiencies, enteral feeding access and the need to avoid the complications associated with overfeeding. The primary aim of this review is to discuss the practicalities of providing nutrition support to those who require it following bariatric surgery, drawing upon published evidence and guidance, while highlighting some of the nutritional challenges in this group of patients. The secondary aim is to highlight gaps in the literature and to identify areas of ambiguity where further research is required.
The types of bariatric surgery performed
There are currently four main weight loss surgical procedures undertaken (see Table 1 for details on the types of bariatric surgery procedures). The specifics of surgery are detailed elsewhere(Reference Santry, Gillen and Lauderdale7–Reference Mognol, Chosidow and Marmuse10). These procedures can be classified as either restrictive or malabsorptive. The only truly malabsorptive procedure is the biliopancreatic diversion with duodenal switch. Currently the Roux en Y gastric bypass (RYGB) is the most commonly performed procedure. This was demonstrated by the Longitudinal Assessment of Bariatric Surgery Consortium study 2009(2), where 71% of the procedures were RYGB, 25% adjustable gastric band and 3% made up from other procedures.
Table 1. Types of bariatric surgery procedures(Reference Santry, Gillen and Lauderdale7–Reference Mognol, Chosidow and Marmuse10)

Complications following bariatric surgery
Adverse effects of the surgery are classified as early, i.e. <30 d and late complications, seen after the first 30 d. Total post-surgical complications or adverse effects following a gastric bypass can be as high as 16%(Reference Nguyen, Silver and Robinson11) (see Table 2 for surgical complications that may result in nutritional consequences). The complications that are likely to be present early are anastomotic leaks, haemorrhages, fistulas and perforations, whereas anastomotic strictures, bowel obstructions and band erosions are more likely to occur after the first 30 d(2, Reference Nguyen, Silver and Robinson11–Reference Gonzalez, Sarr and Smith14).
Table 2. Post-operative complications that result in nutritional consequences(2, Reference Nguyen, Silver and Robinson11–Reference Gonzalez, Sarr and Smith14)
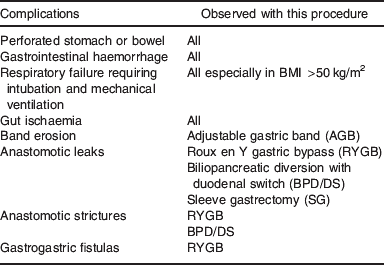
Anastomotic leaks are the most common gastrointestinal complication occurring in 2–5% of laparoscopic gastric bypass cases(Reference Nguyen, Silver and Robinson11–Reference Gonzalez, Sarr and Smith14). Kumpf and co-workers(Reference Kumpf, Slocum and Binkley15) in their survey of nutritional practices with complications post-bariatric surgery reported anastomotic leaks as the most common indication for nutrition support. The leak may occur at the gastro–jejuno and jejuno–jejuno anastomosis as well as at the gastric pouch staple line. The gastro–jejuno anastomosis is the most frequent leakage site(Reference Gonzalez, Sarr and Smith14). Revisional surgery (e.g. when an adjustable gastric band has failed to achieve desired weight loss, therefore progressing to an RYGB) has a much higher leak risk of up to 19%(Reference DeMaria, Sugarman and Kellum12). In addition, those with BMI >50 kg/m2 have a significantly higher overall rate of major complications(Reference Benotti, Wood and Rodriguez16).
These surgical complications may cause nutritional problems as oral intake is often restricted or prohibited until the complication has resolved(Reference Gonzalez, Sarr and Smith14). This imposes the need for enteral or parenteral nutrition (PN) and this can be for a considerable period of time. Clinical experience reveals durations of between 2 and 12 weeks, with 30% of patients being discharged from hospital after complications from bariatric surgery, on some form of artificial nutrition(Reference Kumpf, Slocum and Binkley15).
Pre-operative nutritional status
Prior to instigating any artificial nutritional support, it is essential to ascertain the patient's pre-admission nutritional status, just as one would with a non-obese patient. Obese individuals are assumed to be of good nutritional status, due to their excess energy consumption. Despite this excess, the micronutrient component of the diet may be lacking or deficient(Reference Kaidar-Person, Person and Szomstein17).
Obesity is also associated with an enlarged fatty liver or hepatomegaly(Reference Clain and Lefkowitch18–Reference Papadia, Marinari and Camerini20). Anecdotally, it is reported that it can increase the surgical risk and complexity in patients undergoing laparoscopic surgery(Reference Busetto21–Reference Timar, Sestier and Levey23). Enlarged fatty livers, if damaged during the surgical procedure can rupture and bleed heavily. A large liver also obscures the view of the gastro-oesophageal junction necessary when performing laparoscopic surgery. Hepatomegaly has been cited as the most common cause for conversion to an open procedure from laparoscopic(Reference Schwarts, Drew and Chazin-Caldie24). As a result, many bariatric centres recommend preoperative weight loss as a key component of the pre-operative preparation process(Reference Tarnoff, Kaplan and Shikora25). It can demonstrate the motivation and commitment to surgery, reduction of liver size by 18%(Reference Fris26, Reference Colles, Dixon and Marks27) and abdominal adiposity(Reference Fris26, Reference Colles, Dixon and Marks27), as well as reduce the operating time and length of stay(Reference Huerta, Dredar and Hayden28–Reference Still, Benotti and Wood31). It is has also been demonstrated to be associated with greater weight loss post-operatively(Reference Alger-Mayer, Polimeni and Malone32). The desired weight loss is often achieved through the use of very low energy diets providing between 1883 and 2845 J/d (450 and 680 kcal/d) for 2 to 12 weeks to achieve rapid weight loss(Reference Fris26, Reference Colles, Dixon and Marks27). In some cases, this could result in the loss of lean body mass, increasing the risk of complications related to infections and delayed wound healing.
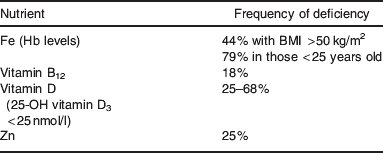
In addition to following the short durations of extreme energy restricted diets, morbid obese patients in general are known to be deficient in many micronutrients(Reference Kimmans, Blanck and Tohill33, Reference Ernst, Thurnheer and Schmid34). It appears that protein and Fe deficiencies increase as BMI does(Reference Ernst, Thurnheer and Schmid34). The most concerning deficiencies in those obese patients awaiting bariatric surgery procedures are Fe, Zn, vitamin B12 and vitamin D(Reference Ernst, Thurnheer and Schmid34–Reference Boylan, Sugerman and Driskell38) (see Table 3 for the incidence of micronutrient deficiencies prior to bariatric surgery). It is anticipated that pre-surgery micronutrient status has been assessed and deficiencies corrected, but this cannot be guaranteed. If the patient has been experiencing intestinal obstruction or anastomotic strictures, vomiting is likely to occur. If prolonged, this may lead to protein energy malnutrition, as well as micronutrient deficiency, leaving the individual with reduced nutritional reserves(Reference Shikora and Tarnoff39).
Table 3. Incidence of micronutrient deficiencies prior to bariatric surgery(Reference Ernst, Thurnheer and Schmid34, Reference Flancbaum, Belsley and Drake35)
Aims of nutrition support in the obese patient
Opposition to nutrition support can be encountered from a range of healthcare workers as well as the patients themselves, the theory being that the avoidance of nutrition support would result in weight loss by promoting the use of energy from excess fat tissues. This assumes that patients have the ability to metabolize the stored fat for energies and conserve protein stores. This concept is yet to be fully proven and puts the patients at high risk of muscle wasting. It is useful to clarify the objectives of nutrition support in these scenarios. The aims of providing nutrition support to obese patients are not dissimilar to those in the non-obese population. These are the avoidance of overfeeding and its harmful effects (e.g. increased CO2 production, which may increase respiratory effort(Reference Talpers, Romberger and Bunce40, Reference Marik and Varon41), promotion of lipogenesis causing hepatic dysfunction(Reference Sheldon, Peterson and Saunders42) and also insulin resistance(Reference Black, Brooks and Bessey43). Preservation of lean body mass and promoting healing is also of paramount importance. It has been observed that malnutrition can occur even in the most obese patients and that it develops quickly when patients are critically ill(Reference Shikora, Johannigma and Civetta44). In this case, it is vital to ensure timely nutrition support. These aims can be at odds with those of the patients. In these circumstances, patients can perceive weight loss as the primary goal of their surgery irrespective of complications. The opportunity to have a period of time without nutrition can be considered as a chance to optimize weight loss further. Time to negotiate mutually acceptable treatment goals is advocated and ensures greater compliance.
In order to avoid the complications associated with overfeeding obese critically ill patients, it is probably sensible to be cautious with total energy and carbohydrate provision. Permissive underfeeding or hypoenergetic feeding with high protein has been proposed by several authors(Reference Burge, Goon and Choban45–Reference Dickerson, Boshert and Kudsk48). It is hypothesized that obese patients can tolerate a reduction in energy, but they still require sufficient protein to maintain lean body mass. Dickerson and co-workers in 2002(Reference Dickerson, Boshert and Kudsk48) reported on a retrospective study of 40 obese critically ill patients. They evaluated the nutritional and clinical efficacies of enteral feeding with hypoenergic, high protein formula v. euenergic high protein. The hypoenergetic group has a significantly reduced intensive care unit stay and decreased duration of antibiotic therapy and a trend towards decreased days on mechanical ventilation. Nitrogen balance was similar between both groups, but was negative during the first 2 weeks of feeding.
Estimated nutritional requirements
There is still a lack of consensus regarding the optimum method for predicting the nutritional requirements for morbidly obese patients requiring nutritional support. The literature suggests that predicting the energy requirements is difficult(Reference Horgan and Stubbs49), with predictive equations being inaccurate(Reference Horgan and Stubbs49, Reference Alves, Moreira da Rocha and Gonzalez50). Indirect calorimetry is considered the gold standard, but is not readily available; therefore, predictive equations are commonly relied upon in the clinical setting.
The most critical factor for use of predictive equations with the acutely unwell obese patient is establishing the most accurate and appropriate weight to use. The problem lies with the body composition in obese and the fact that actual weight does not reflect the amount of body fat, which is metabolically inactive. The resting metabolic rate is mainly dependent upon fat-free mass(Reference Miller and Blythe51, Reference Webb52). However, as weight is gained, it is gained as both fat and lean body mass(Reference Horgan and Stubbs49), but in severe obesity there is a greater proportion of fat tissue deposited(Reference Forbes53, Reference Forbes54). Due to the differences in body composition, it is difficult to know which weight to use in predictive equations. As a result of the larger lean body mass, using ideal body weight is likely to under-estimate energy needs and the use of actual body weight would overestimate as a consequence of the metabolically inactive fat mass. Adjusted body weight has been proposed, on the assumption that the obese have a lean body mass that equates to 25% more than that of the non-obese. This is not a validated method and does not take into consideration the variations in body compositions.
It is possible to accurately determine measured resting energy expenditure (MREE) in obese patients before and after bariatric surgery. Van Gemert et al.(Reference van Gemert, Westerterp and Greeve55) compared the sleeping metabolic rate with predicted using the Westerterp regression formula(Reference Westerterp, Donkers and Fredrix56). Prior to surgery, the predicted sleeping metabolic rate was equal to measured (10 878 J (2600 kcal)), but once weight loss had occurred (at 1 and 3 years respectively) the measured sleeping metabolic rate was below the predicted by 1255 J (300 kcal)/d. The regression equation requires the input of fat-free mass and fat mass, which is not readily available in clinical settings. De Castro Cesar et al.(Reference de Castro Cesar, de Lima and Rasera57) undertook MREE studies in women following RYGB. They found that prior to surgery the Harris Benedict equation (HBE)(Reference Harris and Benedict58) predicted MREE at 106% and it reduced to 103% at 3 months following surgery. It should be noted that these studies were carried out in well free-living participants. These conditions would have been noticeably different from that of the patient group of interest in this review, who would be acutely unwell and experiencing an inflammatory response. It is therefore also necessary to consider the literature investigating MREE in hospitalized obese patients.
There are many equations available to predict the energy expenditure in hospitalized patients, e.g. The HBE(Reference Harris and Benedict58), Ireton-Jones(Reference Ireton Jones and Jones59), Penn State(Reference Frankenfield, Coleman and Alam60) and the Schofield equation(Reference Schofield61). The HBE and Schofield equations have been developed in healthy and non-obese populations, making their use in hospitalized obese inaccurate. The Schofield equation derived in 1985 which is used in the UK, only had 4·5% of study population with a BMI >30 kg/m2, while the HBE was developed in the early 1900s where the incidence of obesity was far less than today. The addition of stress factors are required to make these predictive equations applicable to the clinical setting.
Several studies have assessed effectiveness of predictive equations in obese hospitalized patients. Anderegg et al. (Reference Anderegg, Worrall and Barbour62) compared a range of different prediction methods (HBE, Ireton-Jones, 88 and 105 J/kg (21 and 25 kcal/kg) with MREE in 36 obese hospitalized American adults requiring nutrition support. MREE equated to 85 (sd 71) J/kg (20·4 (sd 5·1) kcal/kg) actual body weight for those being ventilated and 65 (sd 16) J/kg (15·5 (sd 3·9) kcal/kg) actual body weight for those who were not. They found that the HBE with adjusted body weight with stress factors was most frequently able to predict energy expenditure within 10% under or over MREE. However, this was only achieved in 50% of participants. Alves et al. (Reference Alves, Moreira da Rocha and Gonzalez50) found the HBE with actual body weight with no stress factor, to be the best prediction equation compared with MREE when investigating energy expenditure in obese critically ill patients in a Brazilian intensive care unit. The majority of patients in this study had experienced a fistula following bariatric surgery. Frankenfield et al. (Reference Frankenfield, Coleman and Alam60), compared eight different predictive equations in 202 critically ill patients (50% were obese, BMI range 30–112 kg/m2). They found the Penn State equation that incorporates factors for inflammatory response (body temperature and minute ventilation, read from the ventilator not indirect calorimeter), to be the most accurate in the obese patients. It had an accuracy rate (<10% different from measured) in 70% of the young obese and 59% of the elderly obese. In the 2009 American Society for Parenteral and Enteral Nutrition guidelines(Reference McClave, Martindale and Vanek63), it was recommended that energy requirements for those with BMI >30 are calculated as follows; 46–59 J/kg (11–14 kcal/kg) actual body weight or 92–105 J/kg (22–25 kcal/kg) ideal body weight. Unfortunately details are not provided on how these predictions were derived or if validated. Therefore, no single equation can be strongly recommended over the other. However, of the equations reviewed, it would appear that the HBE with an adjusted weight and stress factor or Penn State equations are the most accurate.
Protein requirements
Even less is known regarding the exact protein requirements in the obese or those following bariatric surgery. During the rapid weight loss phase seen following bariatric surgery a state of semi-starvation may develop. There is potential for the loss of fat-free mass to result in protein malnutrition(Reference MacLean, Rhode and Shizgal64). In hospitalized obese patients, 1·5–2 g/kg of ideal body weight has been advocated to maintain positive N balance(Reference McClave, Martindale and Vanek63, Reference Burge, Goon and Choban45–Reference Dickerson, Boshert and Kudsk48). It should be noted that positive N balance was only achieved with PN and not always associated with significant improvements in morbidity and mortality. In normal circumstances following a gastric bypass 1·1 g/kg of ideal body weight is thought to be necessary to maintain lean body mass(Reference Moize, Geliebter and Gluck65). The joint Bariatric Surgery Guidelines(66) from the American Association of Clinical Endocrinologists, The Obesity Society and American Society for Metabolic and Bariatric Surgery, suggest 80–120 g/d for patients following a biliopancreatic diversion with duodenal switch and 60 g/d or more for those with an RYGB.
Additional vitamin and mineral supplementation
Following RYGB and biliopancreatic diversion with duodenal switch additional vitamin and mineral supplementation is required normally from the day of discharge from the hospital. When complications occur and nutrition support is required, there may be a challenge to provide the additional micronutrients. Two hundred per cent of daily requirements for vitamins are recommended as well as 1500–2400 mg/d elemental Ca and 18 mg elemental Fe and up to 50–100 mg for menstruating women(66). The challenge presents in finding suitable liquid preparations that can be placed down enteral feeding tubes or alternatively can be intravenously given. At that time, the blood results may indicate normal levels of these micronutrients, but it is still recommended that supplementation is provided to maintain current levels and compensate for reduced absorption(66). The amount of micronutrient provision in enteral and PN is designed to meet the requirements of healthy adults in approximately 6276–8368 J (1500–2000 kcal) of nutrition. The level of micronutrient supplementation will not be adequate for those who have undergone malabsorptive bariatric surgery.
Routes of nutrition support to those experiencing bariatric surgery complications
Traditionally, the route of feeding in-patients after bariatric surgery that experience complications would be PN, providing complete gut rest allowing anastomotic leaks and fistulas to heal(Reference Gonzalez, Sarr and Smith14). It is often preferred, due to the perceived difficulties with gaining enteral feeding access, such as getting endoscopic examination of the excluded stomach and also the technical challenges posed by the body. In the Alves et al. 2009 study(Reference Alves, Moreira da Rocha and Gonzalez50), those studied who experienced bariatric surgery complications were all fed PN. The enlarged fatty liver that is commonly present in obese patients(Reference Clain and Lefkowitch18–Reference Papadia, Marinari and Camerini20) can complicate PN provision. Within the critical care literature, it is advised that efforts should be made to enterally feed wherever possible as early enteral nutrition is associated with decreased mortality(Reference McClave, Martindale and Vanek63–Reference Doig, Heighes and Simpson67). Despite the perceived challenges, it is possible to provide enteral nutrition support following bariatric surgery(Reference Pasnik, Krupa and Stanowski68, Reference Sarr69) (see Table 4 for enteral feeding access routes).
Table 4. Enteral feeding access routes(Reference Pasnik, Krupa and Stanowski68–Reference Ross, Semrad and Alverdy70)

Weight loss
When the focus of care following bariatric surgery has been on nutrition support, maintaining nutritional status becomes an important goal. The rapid weight loss normally observed in the first 3 months following bariatric surgery may be dramatic as a result of decreased intake and/or malabsorption. Most patients' dietary intake prior to surgery will be markedly in excess of any energy provision during nutrition support; hence, weight loss will naturally occur. In addition to this, the procedures that involve a component of malabsorption, i.e. RYGB and biliopancreatic diversion with duodenal switch will have other mechanisms involved in further rapid weight loss. This dramatic weight loss is at odds with what is normally associated with nutritional support therapy. The amount of weight that can still be lost following surgery, while being artificially fed, is yet to be studied. It is, on the other hand, known that in normal circumstances following surgery a loss of 0·23–0·45 kg/d or 18–45 kg for 3 months post-operatively can be achieved(66). This is based on an energy intake of less than 4184 J/d (1000 kcal/d), which is normal for the first 3 months following surgery.
Conclusions
There is a dearth of published research in the field of nutrition support to those who require it following complications associated with bariatric surgery. As a consequence, the literature on nutrition support provision to obese hospitalized patients was reviewed, discussed and findings extrapolated. To date, there is still a lack of consensus with regard to the most accurate predictive equation to use in the obese hospitalized patient, although the HBE and Penn State equation may be more accurate. Evidence suggests that hypoenergic feeding with adequate protein may offer potential in improving the outcome, but this is yet to be validated through a randomized controlled trial. More research in all aspects of nutrition support to obese patients especially following bariatric surgery is urgently required to help direct clinical practice.
Acknowledgements
The author declares no conflicts of interest. There was no funding relevant to the paper. E.S. is entirely responsible for the paper.






