The incidence of cancer is progressively increasing worldwide(Reference Siegel, Miller and Jemal1), with its attendant rise of healthcare costs(Reference Sullivan, Pramesh and Booth2). Conversely, mortality rates are declining, particularly in western countries(Reference Siegel, Miller and Jemal1). This diverging evidence suggests effective translation into clinical practice of information on cancer cell metabolism acquired by decades of basic research. However, a closer look at the numbers reveals a different clinical scenario.
When considering the most recent statistics, it appears evident that the 5-year relative survival rate for cancer patients by stage at diagnosis did not change much during the past 5–6 years for those patients diagnosed with advanced disease(Reference Siegel, Miller and Jemal1, Reference Siegel, Naishadham and Jemal3). It is undeniable that medical anti-cancer options significantly improved over the past 10–15 years, yet it is also self-evident that they did not translate into improved outcome for advanced cancer patients in the real world, at least as far as survival rate is considered. This reinforces the importance and cost-effectiveness of the implementation of screening programmes and early diagnosis of cancer. Many reasons could explain the challenges still posed by advanced cancer in achieving clinically meaningful outcomes, among which patient-reported outcomes are gaining more relevance. Human cancer cells have shown exceptional molecular heterogeneity and metabolic plasticity even within the same tumour mass(Reference Hölzel, Bovier and Tüting4), which makes difficult even for precision oncology to achieve significant results(Reference Le Tourneau, Delord and Gonçalves5). In this regard, the use of non-selective chemotherapy and radiotherapy is associated with the development of side effects and toxicity, which may preclude the possibility for cancer patients to receive the treatment schedule as originally planned by oncologists. Identification of the preventable/treatable factors associated with reduced tolerance and efficacy of anticancer therapies is of the utmost importance to enhance the tolerance to treatments, optimise the delivery of chemotherapy and reduce its impact on quality of life.
It is now well established that translation of the results of clinical trials into daily practice does not necessarily result in improved outcome. In this regard, Del Paggio et al. showed that expensive therapies are often less efficacious than cheaper treatments(Reference Del Paggio, Sullivan and Schrag6). Also, Davis et al. reported that of the twenty-three drugs with a survival benefit that could be scored with the validated European Society for Medical Oncology-Magnitude of Clinical Benefit Scale tool, only eleven were judged to offer a clinically meaningful benefit(Reference Davis, Naci and Gurpinar7). Beyond the limitations of the presently available medical therapies, consistent and robust evidence indicate that malnutrition is a negative prognostic factor for cancer patients receiving anticancer therapies.
Malnutrition and cachexia in advanced cancer patients
A recent survey showed that most cancer patients at their first medical oncology visit have already a metastatic disease(Reference Muscaritoli, Lucia and Farcomeni8). The prevalence of malnutrition or the risk of malnutrition increases by the stage of the disease and approximately 60 % of advanced cancer patients are affected(Reference Muscaritoli, Lucia and Farcomeni8). Malnutrition in cancer, as assessed by involuntary weight loss >5 % in the previous 3–6 months, is a potent predictor of outcome. Lu et al. showed that weight loss occurring either before or during chemotherapy is associated with worse overall survival(Reference Lu, Yang and Yu9). Highlighting the importance of body weight on patients’ outcome, Patel et al. reported that weight gain in patients with non-small cell lung cancer is associated with a significant improvement of overall survival(Reference Patel, Pereira and Chen10). Similarly, Meyerhardt et al. showed in a large cohort of colorectal cancer patients that weight loss after diagnosis is associated with worse mortality(Reference Meyerhardt, Kroenke and Prado11). The mechanisms by which body weight declines during anticancer therapies could be related to either increased energy expenditure and/or reduced energy and protein intake. Hypermetabolism in cancer patients is not detectable in all cancer patients, but in approximately half of them(Reference Jouinot, Vazeille and Durand12). In contrast, approximately 80 % of cancer patients eat less energies and proteins(Reference Nasrah, Kanbalian and Van Der Borch13) than the levels recommended by the European Society for Clinical Nutrition and Metabolism(Reference Arends, Bachmann and Baracos14). It is, therefore, tempting to postulate that increasing energy and protein intake of cancer patients would result in improved body weight and improved outcome. Unfortunately, higher consumption of protein and energy correlates with greater weight gain, but it is impossible to predict the response to increased nutritional intake when patients are first assessed(Reference Nasrah, Kanbalian and Van Der Borch13). In fact, reduced food intake is only a component within the complex pathogenesis of cachexia, and metabolic changes, particularly increased inflammatory response, determines the metabolic fate, i.e. muscle anabolism or energy dissipation, of ingested nutrients(Reference Baracos, Martin and Korc15).
Although clinically relevant, assessing malnutrition by body weight change only may provide an imprecise assessment of the nutritional status of cancer patients. The main components of body weight, i.e. muscle mass and fat mass, may respond differently to the presence of the tumour and to nutritional intervention and thus differently influence cancer patients’ outcome. Although it is acknowledged that cancer cachexia can be diagnosed by detecting non-volitional weight loss >5 % in the previous 6 months, it is now widely acknowledged that the key feature of cancer cachexia is muscle loss(Reference Fearon, Strasser and Anker16). Sarcopenia, as assessed by computed tomography scan at the level of the third lumbar vertebra, has been consistently demonstrated to predict anticancer treatment toxicities and shorter survival in medical cancer patients(Reference Cespedes Feliciano, Lee and Prado17) as well as increased postoperative complications in surgical cancer patients(Reference Lieffers, Bathe and Fassbender18). The close relationship between muscularity and clinical outcome is also revealed by the concomitant improvement of muscle mass in patients responding to anticancer therapies(Reference Stene, Helbostad and Amundsen19).
Despite solid evidence showing that malnutrition and cachexia are negative predictors of outcome in cancer patients and that shifting from cachexia to non-cachexia improves survival(Reference Kimura, Naito and Kenmotsu20), the causative association between nutrition intervention and better clinical outcome in cancer patients has not been rigorously demonstrated beyond its established role in improving body weight. This lack of evidence is reflected by international guidelines whose recommendations are not usually based on grade A evidence(Reference Arends, Bachmann and Baracos14).
Nutrition support in advanced cancer patients
As previously mentioned, patients with metastatic cancer are frequently malnourished or at risk of malnutrition. Yet, nutrition support is rarely considered in patients receiving anticancer treatment. A potential explanation could be related to the increasing prevalence of overweight and obesity among cancer patients, which may induce oncologists to delay addressing nutrition-related symptoms and weight loss. However, recent data show that weight loss after diagnosis is a negative prognostic factor even for cancer patients at an early stage of disease(Reference Cespedes Feliciano, Kroenke and Bradshaw21). Therefore, nutrition support should be initiated as soon as poor food intake and weight loss develop.
It is acknowledged that robust evidence is not available to strongly recommend nutrition therapy in metastatic cancer patients receiving treatment. However, this could be due to factors not directly related to a possible inherent futility of nutrition in cancer. As an example, many trials included a limited and heterogeneous population, energetic and protein targets were not pre-defined and whether these targets were met in the trials is information rarely available(Reference de van der Schueren, Laviano and Blanchard22). Consequently, the best strategy to implement nutrition therapy in cancer patients remains the conduction of new clinical trials with precisely defined clinical objectives and involving an adequately powered and homogeneous population(Reference de van der Schueren, Laviano and Blanchard22).
Another important factor that should be considered to optimise nutrition therapy in cancer patients is related to their clinical journey. Most metastatic cancer patients show a survival time of months, and in some cases also of years. During this period, nutritional status is challenged according to the catabolic crisis model(Reference Lunney, Lynn and Foley23). In line with this approach, different crises occur during the clinical journey (i.e. psychological discomfort, surgical stress, chemotherapy-induced inflammatory response, mucositis, etc.), each of them negatively affecting nutritional status which does not fully recover after cessation of the crisis (Fig. 1). Consequently, these same crises, i.e. psychological discomfort, surgical stress, the toxicity of chemotherapy, radiotherapy-induced mucositis, among other factors, impact on nutritional status by a variable combination of reduced food intake/increased energy expenditure/reduced anabolic potential. Therefore, the goal of nutrition support in metastatic cancer patients would be to minimise the effects of ‘crisis’ and maximise recovery in between the catabolic events. The two immediate consequences of these central tenets are (i) early initiation of support and (ii) targeted approach addressing the main reason for weight/muscle loss. Strengthening the relevance of this approach, it has been shown that cancer cachexia is a treatable syndrome since cancer patients retain muscle anabolic potentials up to 3 months before death(Reference Prado, Sawyer and Ghosh24). Prevention and treatment of malnutrition and cachexia is thus a long fight which should not be limited to the few weeks of the catabolic crisis, but should be modulated upon patients’ needs for months and even years.
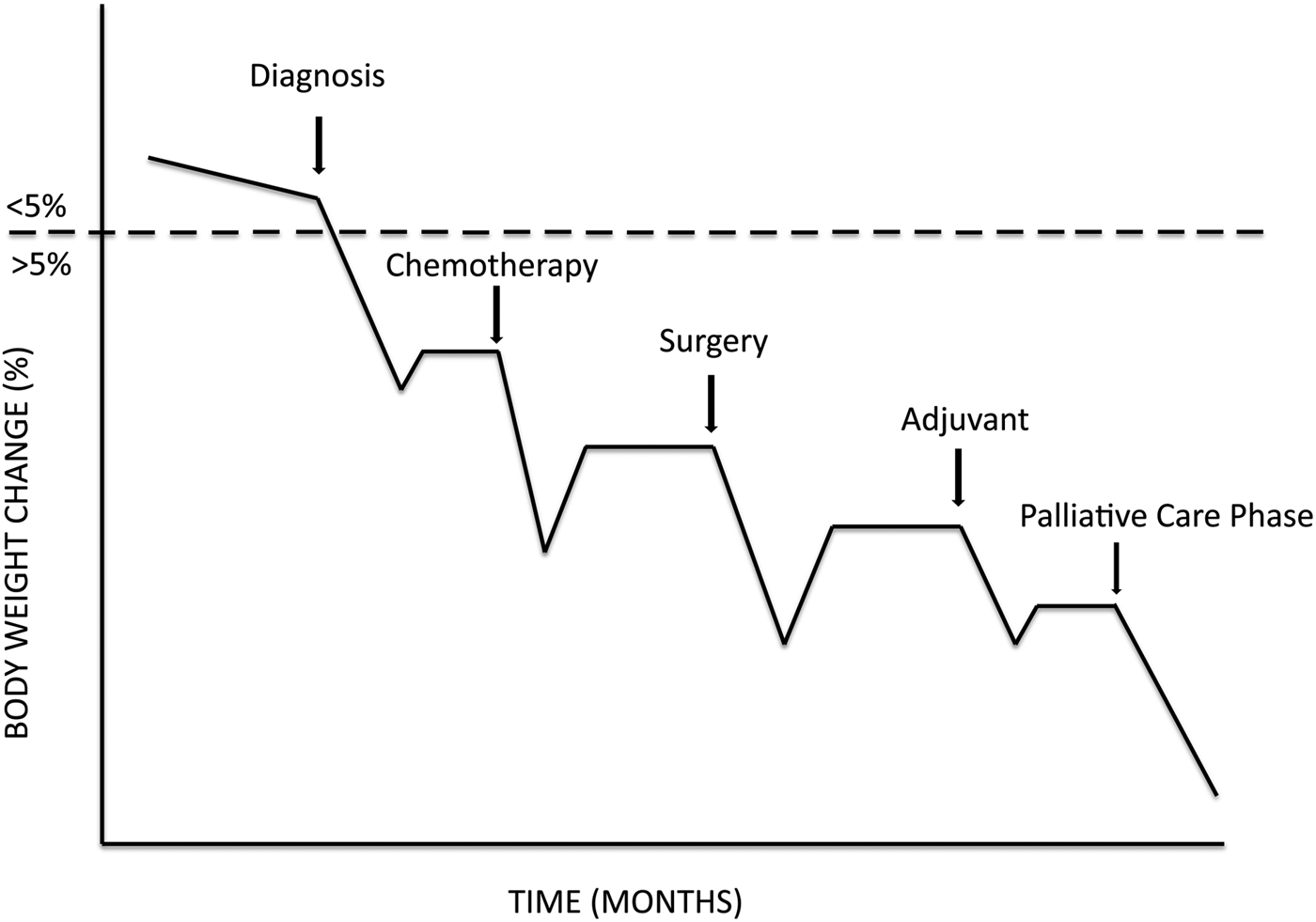
Fig. 1. Catabolic crisis model of the development and progression of cancer cachexia. Disease- and treatment-related events act as triggers of catabolism, thus worsening weight/muscle/function loss. After cessation of the crisis, the recovery phase is almost invariably not exploited to regain the loss and return to baseline levels.
In general, metastatic cancer patients should aim at consuming a quantitative and qualitative adequate diet during their whole clinical journey. The energetic and protein targets for cancer patients have been internationally recognised and are now included in the European Society for Clinical Nutrition and Metabolism guidelines(Reference Arends, Bachmann and Baracos14). When volitional food intake does not meet recommended levels anymore, then artificial nutrition should be considered(Reference Arends, Bachmann and Baracos14). Nutritional counseling and oral nutritional supplements are the first steps of nutrition therapy. If compliance is poor, then enteral nutrition should be considered. If enteral nutrition is not feasible or tolerated, then parenteral nutrition should be initiated. Unfortunately, this decisional chain is frequently not considered by attending healthcare professionals. Data from the European Society for Clinical Nutrition and Metabolism project nutritionDay Oncology, which collects data from real life on nutritional care of cancer patients, show that among 9100 hospitalised cancer patients, oral nutritional supplements are more likely used in the palliative setting (unadjusted OR 1·9 v. cancer patients hospitalised for diagnostic purposes; P < 0·01) rather than in the active treatment period (I Sulz et al.,unpublished results). This highlights the gap between international recommendations and daily practice.
It is acknowledged that the impact of nutrition therapy on the clinical outcome of advanced cancer patients still needs to be precisely assessed. Nevertheless, emerging data from clinical trials suggest a beneficial role. Cox et al. reported a sub-analysis of the results obtained in the SCOPE1 study, an intervention trial assessing the effects of adding or not adding Erbitux to the standard of care in patients with oesophageal cancer(Reference Cox, Powell and Carter25). Authors registered the nutritional risk of enrolled patients and observed that patients at high nutritional risk had a shorter survival(Reference Cox, Powell and Carter25). Interestingly, when patients at higher nutritional risk were stratified according to the nutritional support received (i.e. no intervention, dietary advice, oral intervention, major intervention), those receiving early nutritional care showed a longer survival than patients receiving no intervention(Reference Cox, Powell and Carter25). Although these results are based on a sub-analysis and the numerosity of the population is limited (n 40), the present study suggests that early correction of nutritional impairment may result in a better clinical outcome.
Whether qualitatively adequate diet following cancer diagnosis could improve clinical outcome has been recently investigated in colorectal cancer patients. By assigning an American Cancer Society Nutrition and Physical Activity guidelines score for each patient enrolled in the CALGB 8903/Alliance Trial based on BMI, physical activity and intake of vegetables, fruit, whole grains and red/processed meats, Van Blarigan et al. showed that having a healthy body weight, being physically active and eating a diet rich in vegetables, fruit and whole grains after diagnosis of stage III colon cancer is associated with a longer survival(Reference Van Blarigan, Fuchs and Niedzwiecki26). The specific influence of each nutrient on the observed results is not assessable, yet contrary to the general recommendations, low intake of red and processed meat after colon cancer is associated with an increased risk of death, most likely because low consumption of red meat intake could be considered as a surrogate marker of poor protein intake(Reference Van Blarigan, Fuchs and Niedzwiecki26). This evidence highlights that nutritional guidelines for cancer prevention may not necessarily yield to significant clinical benefit in cancer patients. Also, they underscore the fact that food is a potent inducer of metabolic responses and could be considered as a disease-modifying agent, at least when a few nutrients are included in the diet.
Metabolically active nutrients in advanced cancer patients
Metabolic changes, mainly induced by the increased inflammatory response, are key factors in the pathogenesis of cancer cachexia. Modulation of the inflammatory response may restore the anabolic potential of muscle mass and enhance nutrient-driven protein synthesis. Ultimately, this may result in an improved clinical outcome. n-3 Fatty acids, namely EPA and DHA, have been demonstrated to exert anti-inflammatory effects in experimental models and cancer patients although the results are not conclusive. Therefore, the European Society for Clinical Nutrition and Metabolism guidelines only suggest the use of EPA and DHA to stabilise or improve appetite, food intake, lean body mass and body weight(Reference Arends, Bachmann and Baracos14). We recently performed a systematic review and meta-analysis of clinical trials testing the effects of oral nutritional supplements enriched or not-enriched with n-3 fatty acids on clinical and nutritional outcomes in cancer patients receiving chemotherapy(Reference de van der Schueren, Laviano and Blanchard22). Results obtained show that oral nutrition supplements increase body weight, but this effect is mainly related to the use of n-3 enriched supplements. Modulation of chemotherapy-induced inflammatory response may thus restore anabolic potential of cancer patients, but it may also sensitise cancer cells to the toxic effects of chemotherapy.
Whether the use of n-3 fatty acids may also result in an improved clinical outcome, i.e. tumour response, survival etc., remains a debated issue. Supporting evidence has been published. Song et al. showed that colorectal cancer patients who increase dietary n-3 fatty acid intake after the diagnosis of cancer benefit from a reduction of colorectal cancer-specific death, although overall mortality remains unchanged(Reference Song, Zhang and Meyerhardt27). Similar results have been reported by Van Blarigan et al. (Reference Van Blarigan, Fuchs and Niedzwiecki28). Patients in the highest v. lowest quartile of marine n-3 intake had a hazard ratio (HR) for disease-free survival of 0·72 (95 % CI 0·54, 0·97; P trend = 0·03). Individuals who consumed dark fish (i.e. the main source of fish oil) ≥1/week v. never had longer disease-free survival (HR 0·65; 95 % CI 0·48, 0·87; P = 0·007), recurrence-free survival (HR 0·61; 95 % CI 0·46, 0·86; P trend = 0·007) and overall survival (HR 0·68; 95 % CI 0·48, 0·96; P trend = 0·04). In a subset of 510 patients, the association between marine n-3 fatty acid intake and disease-free survival appeared stronger in patients with high PG-endoperoxide synthase 2 expression (HR 0·32; 95 % CI 0·11, 0·95; P trend = 0·01) compared with patients with absent/low PG-endoperoxide synthase 2 expression (HR 0·78; 95 % CI 0·48, 1·27; P trend = 0·35; P interaction = 0·19). This evidence is of key importance since it shows that the beneficial effects of n-3 fatty acid intake are related not only to the dose consumed with the diet but also with the genetic predisposition to properly metabolise them. In an intervention study, Shirai et al. studied gastrointestinal cancer patients with cachexia and supplemented them during chemotherapy with supplements containing or not containing EPA and DHA(Reference Shirai, Okugawa and Hishida29). No improvement of overall survival was observed in the total cohort, but those patients with the increased inflammatory response at baseline and receiving n-3 fatty acid enriched supplements had a significantly better survival than those with the same inflammatory response but not receiving n-3 fatty acids. This evidence reinforces the key concept that the benefit of nutrition support and metabolically active nutrients are achieved when they are prescribed to the right patient at the right moment.
Challenges ahead
The nihilistic approach of oncologists to cancer cachexia is expected to disappear in future years. More advanced cancer patients with nutritional impairment will be diagnosed, thereby dragging oncologists from their torpor toward nutritional care. The rising importance of patient's reported outcomes will force malnutrition and cachexia into the Oncology agenda. New therapeutic strategies and particularly immunotherapy will benefit from nutritional care and preserved food intake(Reference Nakao, Muramatsu and Kagawa30, Reference Flint, Janowitz and Connell31). However, we should acknowledge that nutritional therapy cannot be considered the magic bullet in the care of advanced cancer patients. Metastatic cancer patients are often multisymptomatic. Effectively addressing only one of these symptoms may not result in a significant clinical benefit. Therefore, the more appropriate care of cancer patients is based on a multimodal approach which includes optimal oncological management and concurrent targeting of the need and expectations of the patients. Of great interest, this simultaneous multidisciplinary and multiprofessional approach has been already demonstrated to yield to statistically significant improvement of the quality of life(Reference Haun, Estel and Rücker32). Early multimodal care has the potential to also enhance survival, but a recent systematic review with meta-analysis could only disclose a non-statistically significant trend(Reference Haun, Estel and Rücker32). However, results from new trials being completed could progressively give statistical power to the role of early palliative care in reducing mortality.
The most difficult challenge ahead is the implementation of nutritional care in daily practice. Although the American Society of Clinical Oncology recommends palliative care involvement for all advanced cancer patients within 8 weeks from diagnosis(Reference Ferrell, Temel and Temin33), it is also acknowledged that compliance by oncologists to nutritional recommendations is suboptimal. In this setting, it is important not only to disseminate data and results, but cancer patients as well should ask for treatment of their accompanying symptoms.
In summary, the clinical journey of an advanced cancer patient may extend over a period of months or years. During this period, nutritional status is challenged by the metabolic disturbances induced by a tumour and by anticancer therapies. Yet, windows of opportunity should be exploited to improve nutritional status, enhance the efficacy of anticancer therapy and possibly improve survival. Meeting energy and protein requirements are key to obtain clinically relevant effects, but compliance is not optimal. The use of metabolically active nutrients may further enhance the efficacy of antitumour therapy. When considering nutrition therapy within the framework of early palliative care, it comes to mind the popular motto that ‘the best advances in Medicine will not come from the discovery of new drugs, but from the more effective use of the existing ones’.
Acknowledgements
The authors wish to acknowledge and thank Dr Ilaria Lospinuso for editorial assistance.
Financial Support
None.
Conflicts of Interest
Dr Laviano received honoraria from nutrition industries for independent lectures during industry-sponsored educational and scientific meetings. Dr Di Lazzaro and Dr Koverech declare no conflict of interest.
Authorship
Dr Laviano devised and wrote the manuscript. Dr Di Lazzaro and Dr Koverech reviewed the relevant literature, updated the reference list, reviewed the manuscript and made substantial additions to the text.



