Six-hundred million adults around the world are suffering from obesity, defined as an abnormal or excessive fat accumulation that may impair health(1). Worldwide, different prospective studies and meta-analyses observed that both overweight, defined as a BMI ≥25·0 kg/m2, and obesity, defined as a BMI ≥30·0 kg/m2, are associated with increased all-cause mortality(Reference Di Angelantonio and Bhupathiraju2). More specific, every 5 kg/m2 increase in BMI above 25 kg/m2 is associated with an average increase in mortality of 30 %. Predominantly, the excess mortality is mainly the result of vascular diseases (e.g. IHD, stroke and other vascular diseases) and diabetes(Reference MacMahon, Baigent and Duffy3). These factors, including the growing prevalence of obesity, the severity of associated comorbidities and the associated economic costs, explain the rising interest in preventive strategies with limited results so far(Reference Bray, Frühbeck and Ryan4). Treatment of obesity is therefore imperative with lifestyle changes, dietary adjustments and increased physical activity as cornerstone(Reference Fried, Yumuk and Oppert5, Reference Mechanick, Youdim and Jones6). Despite the ease of lifestyle modification, whether or not combined with pharmacological treatment, bariatric surgery results in greater and sustained improvements in weight loss, obesity-associated complications, all-cause mortality and quality of life compared with non-surgical treatment options(Reference Sjöström, Narbro and Sjöström7, Reference Sjöström8). These encouraging results explain the worldwide rising number of bariatric procedures as roughly half a million bariatric procedures were performed in 2013(Reference Angrisani, Santonicola and Lovino9).
Metabolic and bariatric surgery
Bariatric procedures are intended for patients suffering from morbid obesity where cornerstone treatment (e.g. lifestyle changes, dietary adjustments and increased physical activity) and/or pharmacological treatment produces insufficient weight loss. European and American guidelines recommend bariatric surgery for patients with a BMI ≥40 kg/m2 or for patients with a BMI ≥35 kg/m2 in combination with at least one obesity-associated comorbidity (e.g. type 2 diabetes, hypertension or obstructive sleep apnoea)(Reference Fried, Yumuk and Oppert5, Reference Mechanick, Youdim and Jones6). Traditionally, bariatric surgery procedures are classified as restrictive, malabsorptive or a combination thereof. Restrictive procedures reduce the size of the stomach, which limits the energetic intake and triggers satiety, while malabsorptive procedures bypass a specific part of the intestine, which impedes the absorption of the ingested nutrients in the gastrointestinal tract. Combined procedures include both the aspects of restriction and malabsorption(Reference DeMaria10). The positive effects of bariatric surgery extend beyond weight loss as the metabolic status of the patients is drastically improved after surgery. Accordingly, the concept of metabolic and bariatric surgery has emerged and gained acceptance over the years(Reference Rubino and Cummings11, Reference Rubino12). For the remainder of the review, metabolic and bariatric surgery will be referred to as bariatric surgery.
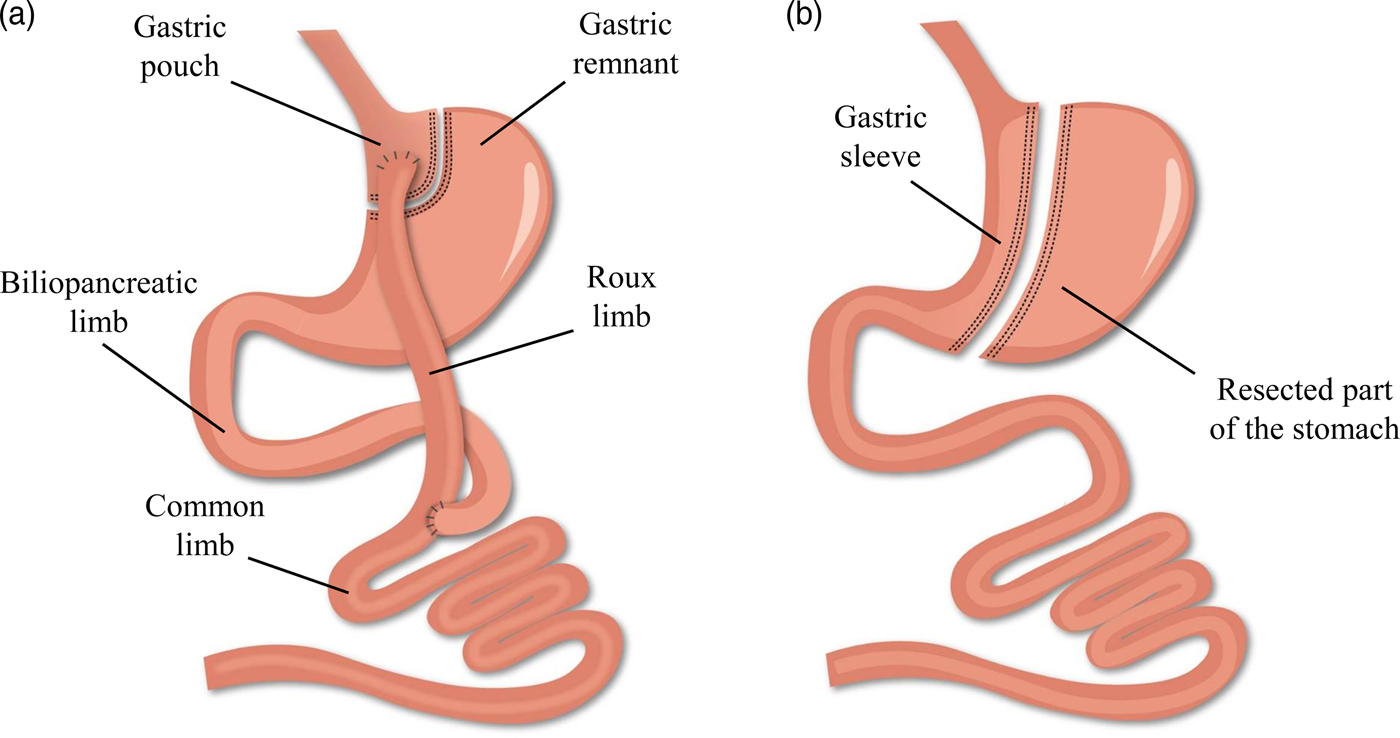
While bariatric surgery is established to induce weight loss and/or improve the metabolic profile, several evolutions have occurred within the anatomical procedures(Reference Buchwald13). Worldwide, the combined Roux-en-Y gastric bypass (RYGB) procedure is considered the gold standard of all bariatric procedures in view of its beneficial balance between the long-term efficacy and complication rate. However, the more recent, restrictive sleeve gastrectomy (SG) procedure is rapidly gaining popularity and has exceeded RYGB as the most commonly performed procedure within academic medical centres of the USA(Reference Esteban Varela and Nguyen14). The alterations in the gastrointestinal anatomical architecture after RYGB and SG are visualised in Fig. 1. Briefly, the laparoscopic RYGB procedure involves the formation of a small gastric pouch and a gastric remnant using surgical staples. Afterwards, the small intestine is rearranged into a Y-configuration through the segmentation of the proximal part of the small intestine distal to the ligament of Treitz. The distal part of the small intestine is reconnected to the small gastric pouch through a gastrojejunostomy with the formation of the Roux limb, while the proximal part of the small intestine is reconnected to the Roux limb through a jejunojejunal anastomosis to facilitate the passage of secreted digestive enzymes and bile salts. The laparoscopic SG procedure involves the resection of the greater curvature of the stomach starting at the antrum between the pylorus and the end of the nerve of Latarjet up to the angle of His. A sleeve-like pouch is formed that connects the oesophagus to the small intestine(Reference Vidal, Corcelles and Jiménez15, Reference Lannoo and Dillemans16). These restrictive and malabsorptive alterations in the gastrointestinal anatomical architecture have a direct effect on the intake, digestion and absorption of nutrients, while additionally inducing changes in the levels of several gut peptides involved in the regulation of appetite and satiety. Taken together, both anatomical and physiological alterations contribute to the desired beneficial weight and metabolic effects(Reference Ionut, Burch and Youdim17).
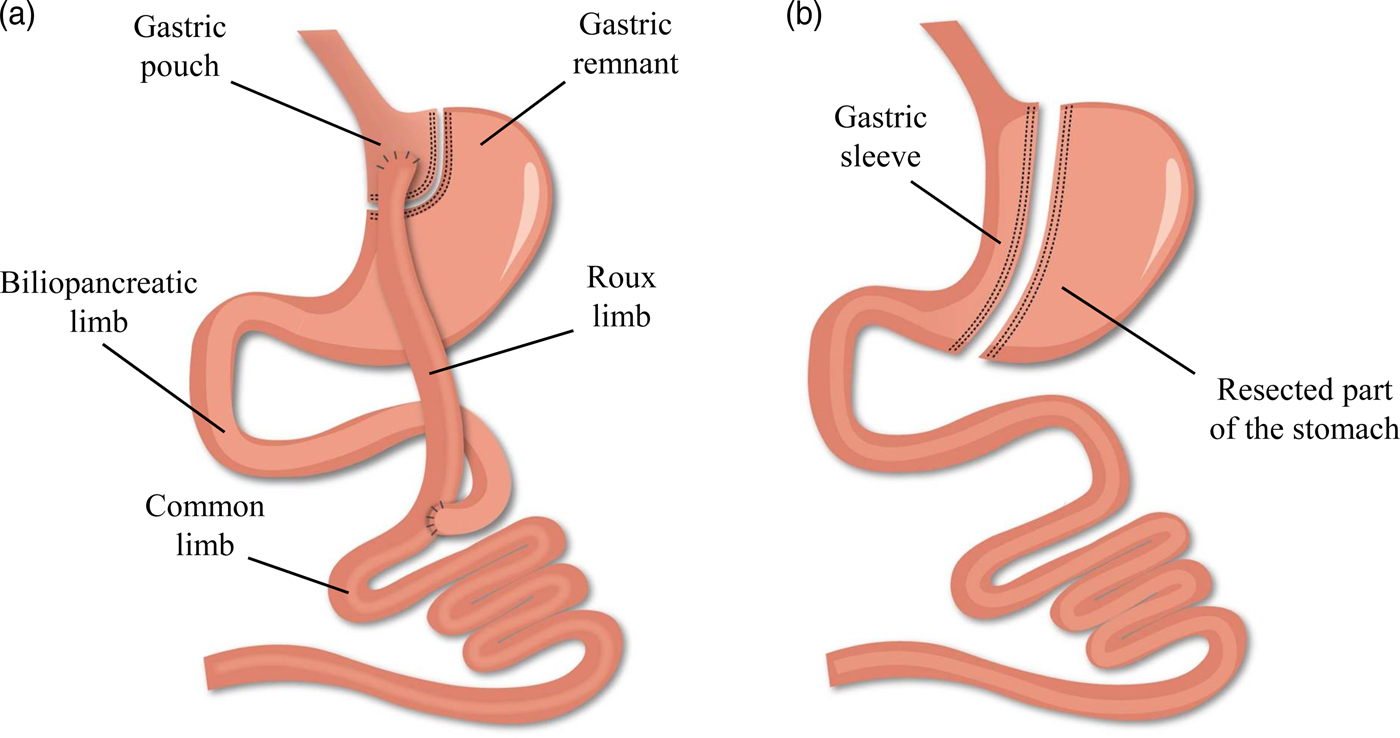
Fig. 1. (Colour online) Alterations in the gastrointestinal anatomical architecture after Roux-en-Y gastric bypass (a) and sleeve gastrectomy (b).
Iron deficiency after bariatric surgery
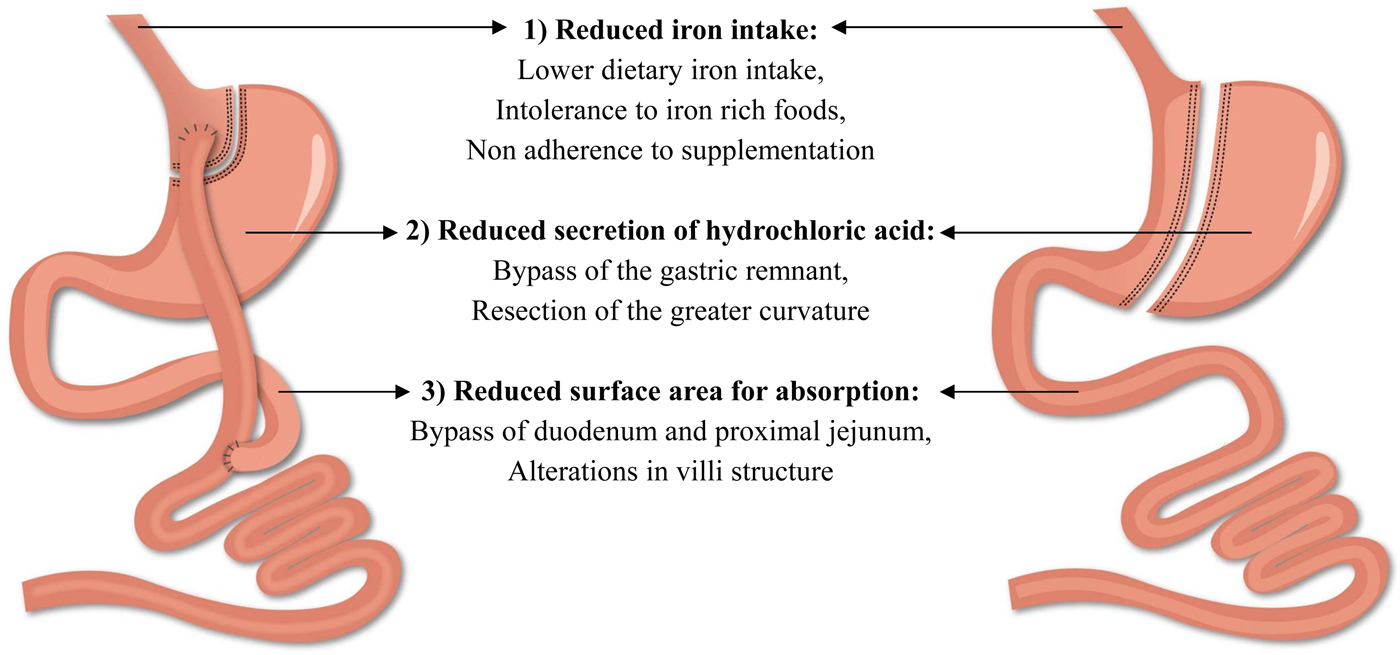
The beneficial results of bariatric surgery with respect to weight loss and comorbidities come at a cost, namely the risk for postoperative complications. Among all complications, the frequency of nutritional complications is a worrying trend and clearly demands extra attention(Reference Decker, Swain and Crowell18). These deficiencies develop as a consequence of the alterations in the gastrointestinal anatomical architecture and the associated changes in the physiology of the gastrointestinal tract(Reference Steenackers, Gesquiere and Matthys19). Particularly striking is the risk of developing iron deficiency as it impairs the normal physiological function of tissues such as blood, brain and muscles(20). Different factors contribute to the development of iron deficiency after bariatric surgery including reduced iron intake, reduced secretion of hydrochloric acid and a reduction in the surface area for absorption (Fig. 2). Taken together, these factors contribute to the development of iron deficiency after bariatric surgery and will be further discussed in the review(Reference Steenackers, Gesquiere and Matthys19, Reference Gesquiere, Lannoo and Augustijns21). Furthermore, it should be emphasised that obese patients are already predisposed to develop iron deficiency. Inadequate iron intake, greater requirements due to a higher blood volume and the presence of low-grade chronic inflammation inhibits the absorption of iron, which may lead to iron deficiency in obese patients and can then persist or even worsen after bariatric surgery(Reference Aigner, Feldman and Datz22).
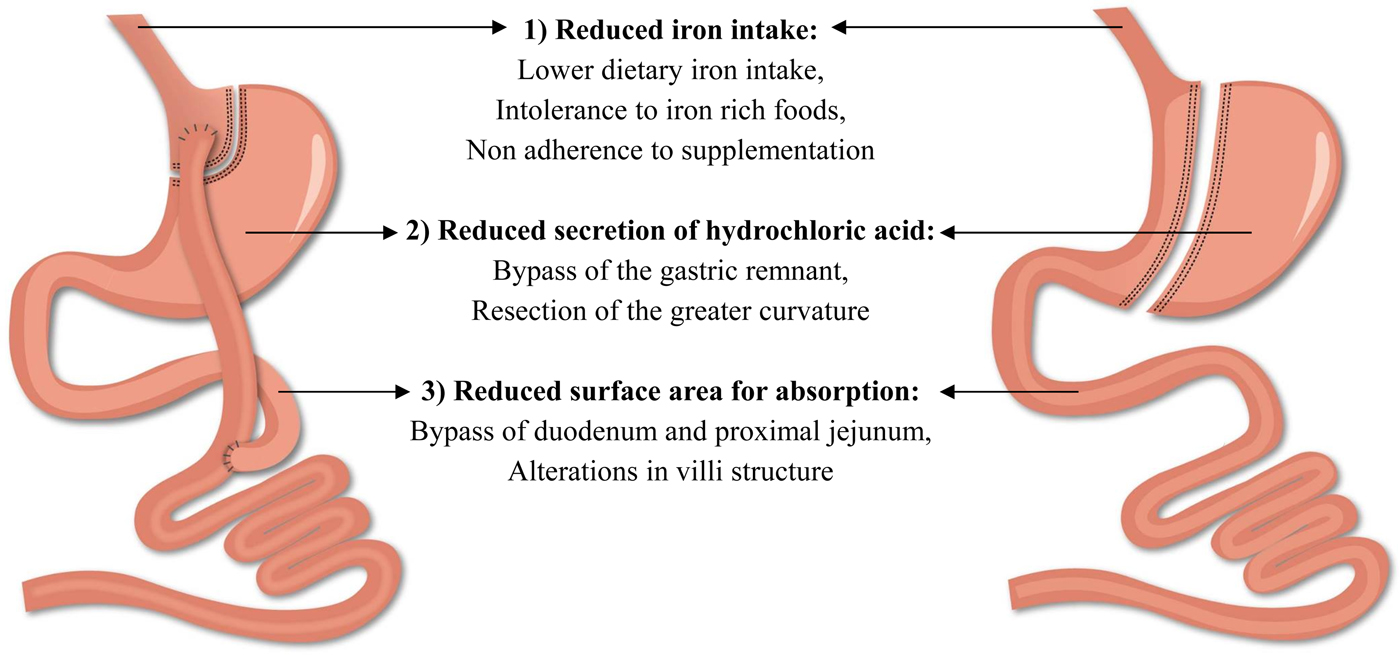
Fig. 2. (Colour online) Factors contributing to the development of iron deficiency after Roux-en-Y gastric bypass and sleeve gastrectomy.
Iron intake after bariatric surgery
The contribution of iron intake to iron deficiency after bariatric surgery has been the topic of investigation in several studies. Tables 1 and 2 provide an overview of the studies to date that have evaluated iron intake after RYGB and SG. After surgery, iron intake was mostly lower or even inadequate compared with the available estimated average requirements or dietary reference intake for healthy individuals(Reference Colossi, Casagrande and Chatkin23–Reference Chou, Lee and Almalki35). Postoperatively, the restricted dietary intake, increased satiety and reduced appetite contribute to the lower iron intake by means of a reduced intake of micronutrients(Reference Moizé, Andreu and Flores27). Furthermore, the lower iron intake is partially the result of the low tolerance for red meat. Recent studies report a rate of intolerance ranging from 23 to 50 % after bariatric surgery. Differences in reporting methodology explain the variety in the reported prevalence. For instance, disparity within the definition for red meat intolerance is observed between the different studies. Nicoletti et al. defined red meat intolerance ‘as an abnormal physiologic response (nausea and vomiting) after eating red meat’, while Moize et al. defined red meat intolerance as ‘nausea, vomiting, diarrhoea or abdominal discomfort following the ingestion of red meat’. Despite this variance, the tolerance for red meat improves as time passes further from the bariatric procedure(Reference Nicoletti, de Oliveira and Barbin36, Reference Moize, Geliebter and Gluck37). Finally, poor adherence to dietary guidelines provided by professionals, non-adherence to recommended supplementation or insufficient professional guidance further contribute to low iron intake. Adherence to the postoperative dietary recommendations has been reported to be inadequate, which tends to increase over time. Supplementation adherence tends to be lower in the late postoperative period compared with the early postoperative period(Reference Hood, Corsica and Bradley38). Concerning professional guidance, accreditation standards from the American Society for Metabolic and Bariatric Surgery (ASMBS) and the International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO) recommend that patients receive postoperative follow-up(Reference Melissas, Torres, Yashkov and Agrawal39, Reference Saber, Bashah, Zarabi and Agrawal40). Nonetheless, it has been reported that 47–90 % of patients do not receive nutritional advice after surgery(Reference Peacock, Schmidt and Barry41).
Table 1. Overview of studies reporting iron intake after Roux-en-Y gastric bypass
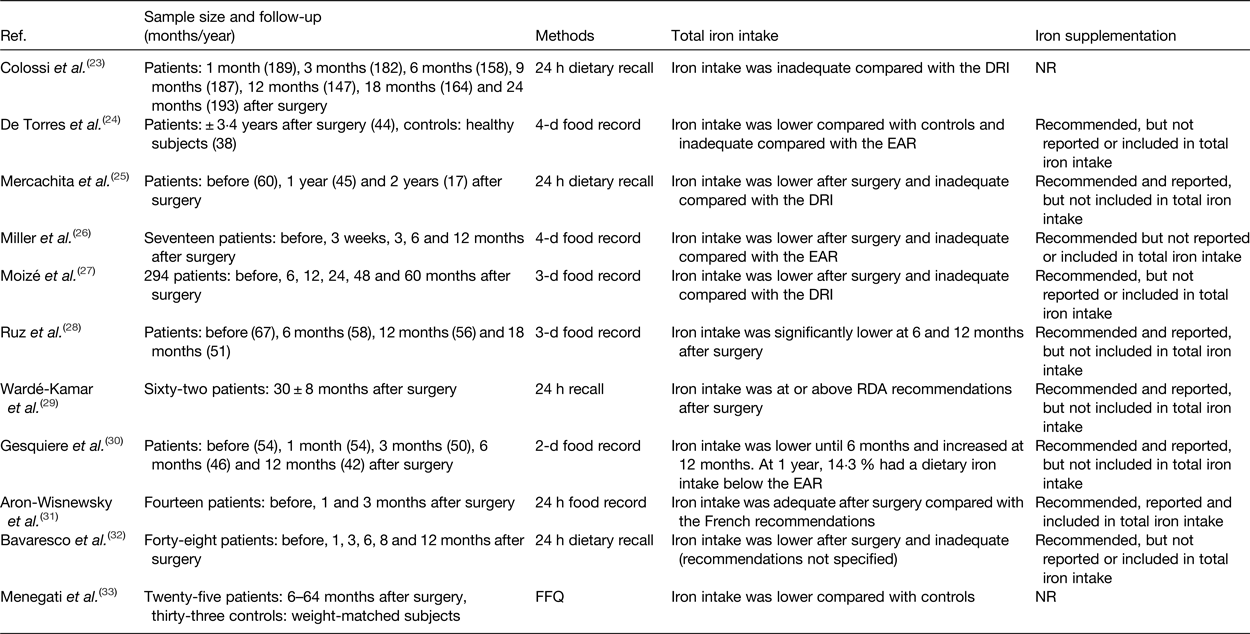
Ref., reference; NR, not reported; DRI, dietary reference intake; EAR, estimated average requirements.
Table 2. Overview of studies reporting iron intake after sleeve gastrectomy
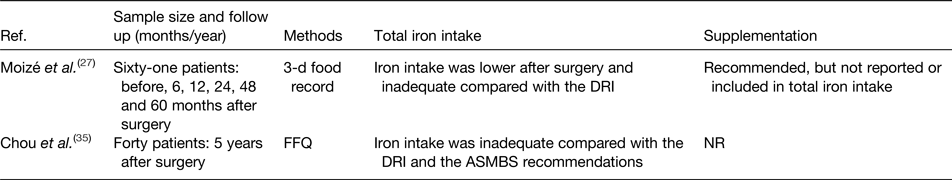
Ref., reference; NR, not reported; DRI, dietary reference intake; ASMBS, American Society for Metabolic and Bariatric Surgery.
Iron absorption after bariatric surgery
Low iron intake after surgery is not an exclusive explanation for the development of iron deficiency. The digestion and absorption of iron are affected by alterations in the gastrointestinal anatomical architecture as illustrated in Fig. 2. First, the reduced secretion of hydrochloric acid hinders the reduction of ferric iron into the absorbable ferrous form(Reference Ishida, Faintuch and Paula42, Reference Behrns, Smith and Sarr43). Secondly, the bypass of the duodenum and proximal part of the jejunum after RYGB reduces the intestinal absorption area for iron, which is mainly absorbed in the duodenum. Thirdly, the villi of the gastrointestinal tract are affected and potentially further contribute to the reduction of the intestinal absorption area for iron after surgery. Spak et al. and Casselbrant et al. found a decrease in villi height in the Roux limb after RYGB, while no studies have investigated potential changes in villi height after SG(Reference Spak, Björklund and Helander44, Reference Casselbrant, Elias and Fändriks45). Taken together, bariatric surgery impedes the ability to absorb iron from both dietary sources and nutritional supplementation. To the best of our knowledge, five studies investigated the impact of bariatric surgery on iron absorption by comparing pre- and postoperative data(Reference Gesquiere, Lannoo and Augustijns21, Reference Ruz, Carrasco and Rojas28, Reference Ruz, Carrasco and Rojas46–Reference Rosa, De Oliveira-Penaforte and De Arruda Leme48). Ruz et al. investigated the absorption of dietary iron after RYGB (n 36). Iron absorption tests were performed before and at 6, 12 and 18 months after RYGB using a standard diet containing 3 mg labelled iron. At each follow-up, iron absorption was significantly decreased to approximately 30 % of the preoperative baseline value(Reference Ruz, Carrasco and Rojas28). To distinguish between haem- and non-haem iron, iron absorption tests were performed before and 12 months after RYGB (n 20) and SG (n 20) using a standard diet containing labelled ferric chloride and labelled haem iron. Iron absorption from both haem and non-haem iron decreased significantly after RYGB and SG, but the magnitude of reduced absorption for haem iron was greater compared with non-haem iron (haem absorption: 23·9 (sem 22·2–25·8)% v. 6·2 (sem 5·3–7·1)%; non-haem absorption: 11·1 (sem 9·8–12·5)% v. 4·7 (sem 3·1–5·5)%)(Reference Ruz, Carrasco and Rojas46).
In addition to the absorption of dietary iron, the alterations in the gastrointestinal anatomical architecture potentially interfere with the dissolution and absorption of iron supplements. In 1999, Rhode et al. investigated the absorption of 50 mg ferrous gluconate 3·2 years after RYGB (n 55). After surgery, 65 % of the included patients appeared to have normal iron absorption, defined as more than 100 % change in serum iron concentration over 3 h after administration. In the patients with normal absorption, a higher incidence of anaemia and lower levels of ferritin concentration were observed(Reference Rhode, Shustik and Christou47). Additionally, two studies investigated the response to iron sulphate. Rosa et al. performed iron tolerance tests with 15 mg elemental iron originating from ferrous sulphate before and at 3 months after RYGB (n 9). Despite a delayed response in the first hour, no significant differences were observed in iron response after surgery, although six of the nine patients demonstrated a mean decrease of 50 % in area under the curve(Reference Rosa, De Oliveira-Penaforte and De Arruda Leme48). Nonetheless, Gesquiere et al. performed iron challenges with 100 mg ferrous sulphate in iron-deficient patients after RYGB (n 23). One patient had sufficient absorption, defined as an increase in serum iron concentration larger than 80 µg/dl(Reference Gesquiere, Lannoo and Augustijns21). Based on these three studies, it is impossible to compare the level of iron absorption due to differences in study design, type of iron, dosage and formulation of the supplement. Iron absorption studies assessing the erythrocyte incorporation using stable iron isotopes would provide more convincing data on the effectiveness of iron supplementation after bariatric surgery(Reference Wienk, Marx and Beynen49).
Iron status after bariatric surgery
In light of the risk to develop iron deficiency, different studies have examined the preoperative and postoperative nutritional status of iron after RYGB and SG. Postoperatively, the prevalence of iron deficiency varies between 18·0 and 53·3 % during a follow-up of maximal 11·6 years after RYGB, while the prevalence of anaemia ranges between 6·0 and 63·6 %(Reference Gesquiere, Foulon and Augustijns30, Reference Aron-Wisnewsky, Verger and Bounaix31, Reference Salgado, Modotti and Nonino50–Reference Von Drygalski, Andris and Nuttleman68). To elucidate the contribution of iron deficiency to the development of anaemia, two studies evaluated the prevalence of iron deficiency anaemia during a follow-up of maximal 10 years after RYGB. Within these studies, the prevalence of iron deficiency anaemia ranged between 6·6 and 22·7 % after RYGB(Reference Obinwanne, Fredrickson and Mathiason56, Reference Rojas, Carrasco and Codoceo67). In comparison, the prevalence of iron deficiency varies between 1 and 54·1 % during a follow-up of maximal 5 years after SG. Additionally, the prevalence of anaemia ranges between 3·6 and 52·7 % after SG(Reference Gjessing, Nielsen and Mellgren69–Reference Aarts, Janssen and Berends79). According to a prospective cohort study of Hakeam et al., iron deficiency anaemia occurred in 1·6 % of the patients 1 year after SG(Reference Hakeam, O'Regan and Salem71). Remarkably, the prevalence of iron deficiency, anaemia and iron deficiency anaemia varies widely both after RYGB and SG. Inconsistency within the definition of deficiencies and differences in bariatric procedure, follow-up, dietary guidance and nutritional supplementation clarify the extent of variation.
Diagnosis of iron deficiency, anaemia and iron deficiency after bariatric surgery
One of the major challenges to diagnose patients as iron deficient concerns the definition of iron deficiency. The proportion of patients affected by iron deficiency depends on the proposed iron status markers and their reference ranges. Malone et al. investigated the proportion of patients affected by iron deficiency after RYGB using three different definitions (n 125). First, iron deficiency was defined as serum ferritin <15 ng/ml (male) or <12 ng/ml (female) or as transferrin saturation (TSAT) <20 % (male) or <16 % (female). Secondly, iron deficiency was based on a combination of serum iron <40 µg/dl and serum ferritin <35 ng/ml. Thirdly, iron deficiency was based on serum ferritin <20 ng/ml. Based on the first definition, 28·3 % of the ferritin values and 47·8 % of the TSAT values fulfilled the criteria for iron deficiency. Based on the combination of serum iron and ferritin, 57·5 % of the patients were suffering from iron deficiency, while 43·4 % of the patients were suffering from iron deficiency, based on the third definition(Reference Malone, Alger-Mayer and Lindstrom80). These data strengthen the clinical significance of combining different iron status markers to assess the prevalence of iron deficiency.
In Table 3, an overview of the iron status markers and cut-off values used in the afore-mentioned studies is given for iron deficiency, anaemia and iron deficiency anaemia(Reference Gesquiere, Foulon and Augustijns30, Reference Aron-Wisnewsky, Verger and Bounaix31, Reference Salgado, Modotti and Nonino50–Reference Aarts, Janssen and Berends79). Low levels of serum iron concentration are frequently used to diagnose iron deficiency after surgery(Reference Skroubis, Kouri and Mead61, Reference Hakeam, O'Regan and Salem71, Reference Zarshenas, Nacher and Loi72, Reference Ben-Porat, Elazary and Goldenshluger76, Reference Damms-Machado, Friedrich and Kramer77, Reference Aarts, Janssen and Berends79). However, serum iron concentration is not an absolute diagnostic marker for iron deficiency due to its diurnal variation and external influences(Reference Auerbach and Adamson81). Additionally, diagnosis of iron deficiency is sometimes merely based on serum ferritin concentration(Reference Aron-Wisnewsky, Verger and Bounaix31, Reference Kotkiewicz, Donaldson and Dye51, Reference Kim, Kim and Kwon54, Reference Obinwanne, Fredrickson and Mathiason56, Reference Dogan, Homan and Aarts60). Low levels of serum ferritin imply the presence of depleted iron stores. Nonetheless, concentration of ferritin might be increased as inflammation increases the production of this acute phase protein. As a result, measuring inflammatory markers (e.g. C-reactive protein or α1-acid glycoprotein) could help to interpret ferritin measurements(82, Reference Zimmermann83). Another marker regularly used as a standalone determinant of iron deficiency is TSAT, which is calculated by dividing serum iron concentration with serum transferrin concentration(Reference Ledoux, Calabrese and Bogard58, Reference Coupaye, Puchaux and Bogard62, Reference Karefylakis, Näslund and Edholm65, Reference Vargas-Ruiz, Hernández-Rivera and Herrera66, Reference Al-Sabah, Al-Mutawa and Anderson70). Again, low TSAT is not an absolute marker, but is characteristic for iron deficiency(Reference Auerbach and Adamson81). Which iron status markers should then be used to diagnose iron deficiency after bariatric surgery? As no single iron status marker is an absolute determinant of iron deficiency, the preferred screening approach is a combination of markers. These markers should include at least ferritin concentration, which is relevant in the absence of underlying inflammation, and TSAT, which provides more reliable information in the presence of underlying information. Furthermore, assessing hepcidin and/or the soluble transferrin receptor concentration would provide additional important information regarding iron homeostasis. Nonetheless, the utility of these markers may somehow be limited in clinical practice due to associated costs and availability issues(Reference Ferguson, Skikne and Simpson84–Reference Macdougall, Malyszko and Hider86).
Table 3. Iron status markers and cut-off values used to diagnose iron deficiency, anaemia or iron deficiency anaemia
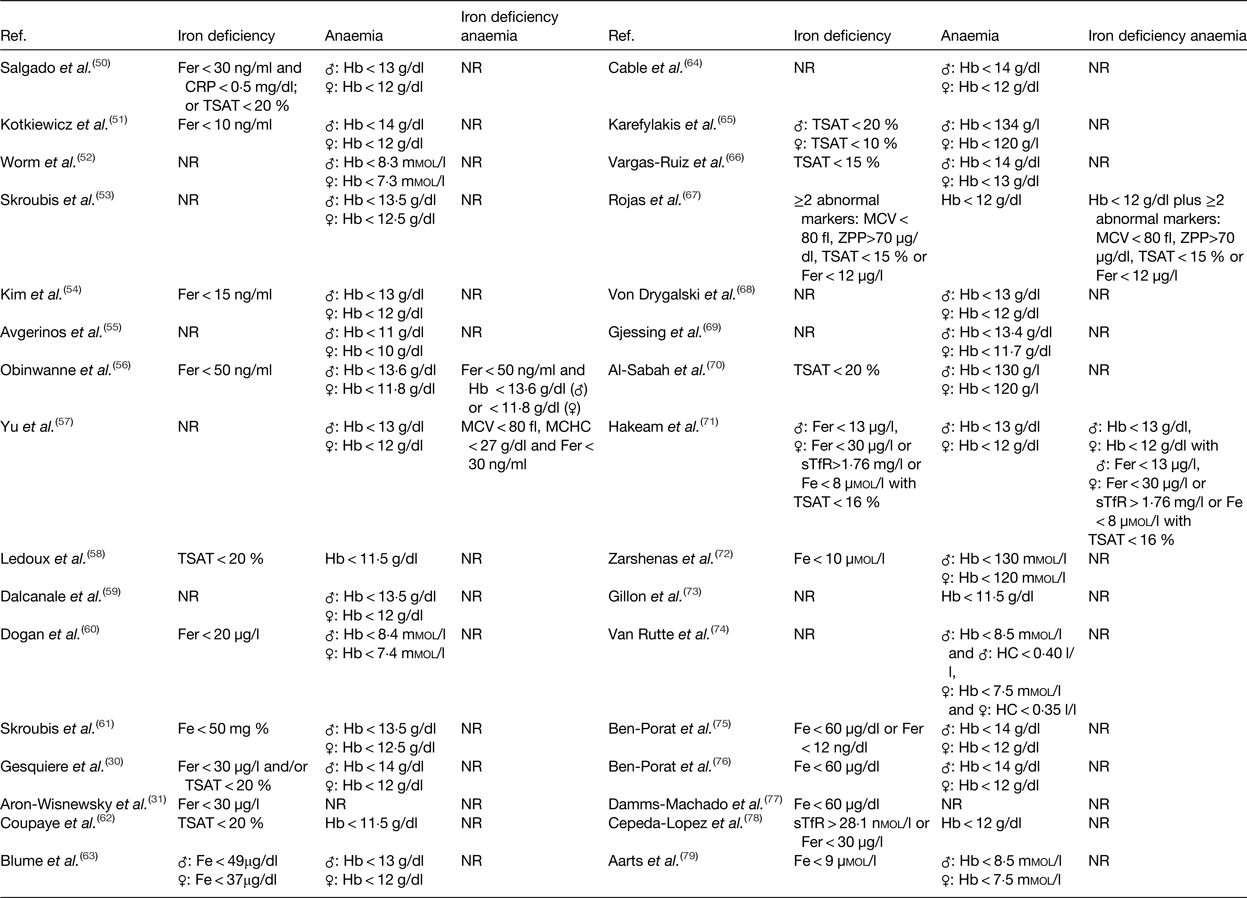
Fer, ferritin; CRP, C-reactive protein; TSAT, transferrin saturation; NR, not reported; MCV, mean corpuscular volume; MCHC, mean corpuscular Hb concentration; Fe, iron; ZPP, zinc protoporphyrin; sTfR, soluble transferrin receptor.
In contrast to diagnosing iron deficiency, one marker is sufficient to detect the presence of anaemia. According to the WHO, Hb concentration below 130 g/l for men aged 15 years or above and a concentration below 120 g/l for non-pregnant females aged 15 years or above is representative for anaemia(87). Although the credibility of these cut-off values has been discussed in literature in light of differences in ethnicity. Therefore, new age, sex and ethnic-specific cut-off values have been proposed(Reference Beutler and Waalen88, Reference Johnson-Spear and Yip89). The disparity in cut-off values for diagnosing anaemia could explain the variety in reference ranges used within the afore-mentioned studies as illustrated in Table 3(Reference Gesquiere, Foulon and Augustijns30, Reference Aron-Wisnewsky, Verger and Bounaix31, Reference Salgado, Modotti and Nonino50–Reference Aarts, Janssen and Berends79). As stated earlier, the prevalence of anaemia reaches an upper limit of approximately 50 % after SG and 65 % after RYGB. These high rates of anaemia may reflect a variety of vitamin or mineral deficiencies, but are predominantly the result of iron deficiency. To distinguish between the different causes, the mean corpuscular volume of erythrocytes can be measured. Microcytic and hypochromic erythrocytes are considered the hallmark finding of iron deficiency anaemia(Reference Zimmermann83, Reference Cook and Finch90). Clearly, there is a need to standardise the definition of iron deficiency, anaemia and iron deficiency anaemia based on the most relevant iron status markers and their reference range.
Nutritional recommendations after bariatric surgery
Throughout the years, different organisations have published guidelines on clinical and nutritional guidance of patients before and after bariatric surgery. Table 4 summarises the available guidelines and updates proposed by the ASMBS and the IFSO concerning nutritional screening for deficiencies, preventive postoperative vitamin and mineral supplementation and postoperative supplementation for the treatment of iron deficiency. To prevent iron deficiency, pre- and postoperative nutritional screening in combination with multivitamin and mineral supplementation is recommended. In case of iron deficiency, oral or parenteral iron administration is required. To rate the quality of their recommendations, both organisations adopted grading systems (IFSO: Oxford centre for evidence-based medicine classification system; ASMBS: the protocol for standardised production of clinical practice guidelines provided by the American Association of Clinical Endocrinologists). However, these grading systems illustrate the lack of strong evidence supporting the preventive postoperative vitamin and mineral supplementation guidelines. In Table 4, the quality of evidence supporting the current recommendations for nutritional screening for deficiencies, preventive postoperative vitamin and mineral supplementation and postoperative supplementation for the treatment of iron deficiency is provided(Reference Fried, Yumuk and Oppert5, Reference Mechanick, Youdim and Jones6, Reference Fried, Hainer and Basdevant91, Reference Mechanick, Kushner and Sugerman92). The absence of strong evidence might also explain the difficulty of patients to adhere to the proposed guidelines as patients experience clinical burden despite preventive strategies. According to a recent study in 16 620 French post-bariatric patients, the number of patients that received reimbursement for a nutritional iron supplement decreased significantly in the first 5 years after surgery(Reference Thereaux, Lesuffleur and Paita93). These data confirm the poor adherence to the international guidelines and advocate for more nutritional research in order to provide strong evidence-based guidelines after bariatric surgery.
Table 4. Nutritional guidelines after bariatric surgery
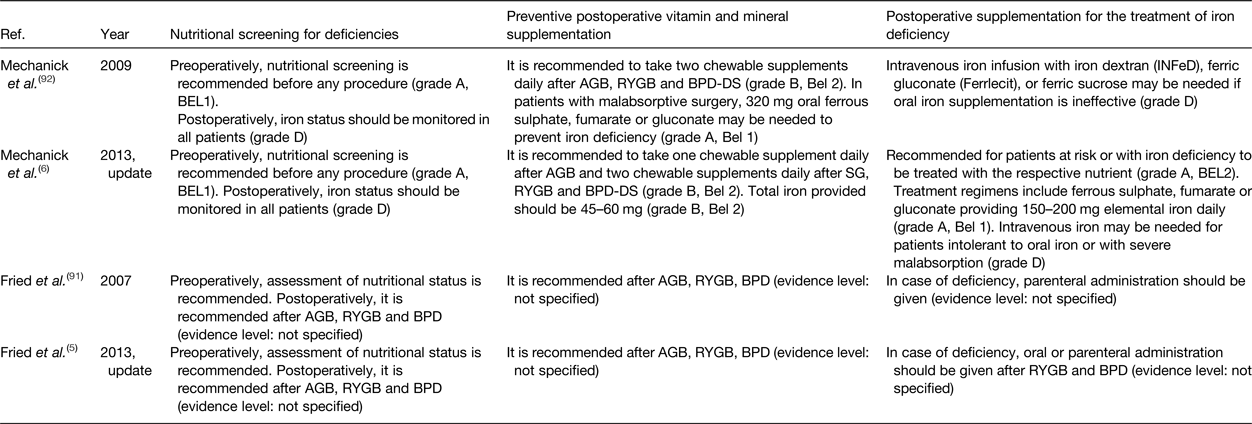
AGB, adjustable gastric banding; RYGB, Roux-en-Y gastric bypass; BPD, bilio-pancreatic diversion; BPD-DS, BPD with duodenal switch; SG, sleeve gastrectomy.
Apart from the guidelines of international organisations, some researchers have provided updates or even proposed new guidelines. For instance, Parrott et al. updated the recommendations of the ASMBS by providing additional guidelines to prevent iron deficiency for patients at low risk, menstruating females and patients after RYGB, SG or biliopancreatic diversion with duodenal switch and by providing treatment guidelines for patients suffering from iron deficiency after bariatric surgery. Furthermore, the authors made recommendations for the dosing of supplements and recommendation on how to avoid potential interactions with other nutrients or medication(Reference Parrott, Frank and Rabena94). Additionally, Dagan et al. proposed nutritional guidelines for vegetarian and vegan patients undergoing bariatric surgery based on their clinical experience and the current knowledge in the field of nutrition in bariatric, vegetarian and vegan patients(Reference Sherf-Dagan, Hod and Buch95). All these different guidelines highlight the heterogeneity in the reporting of bariatric research. Therefore, the ASMBS published outcome reporting standards in 2015. In addition to improving the quality of reporting, these standards are proposed to lower the heterogeneity in outcome reporting within the field of metabolic and bariatric surgery literature. These reporting standards provide consistency and uniformity for authors on how to report the following outcomes: follow-up, diabetes, hypertension, dyslipidaemia, obstructive sleep apnoea, gastroesophageal reflux disease, complications, weight loss and quality of life(Reference Brethauer, Kim and Chaar96). However, reporting standards are missing for the diagnosis and treatment of nutritional deficiencies after bariatric surgery.
Future perspectives
To improve future evidence, a standardised reporting methodology should be included for various important aspects of bariatric research. For instance, clinical outcomes of patients regarding nutritional deficiencies have been compared in review papers and meta-analyses without accounting for differences in screening and treatment approaches. This matter leads to heterogeneity in the interpretation of similar studies. Therefore, a standardised reporting methodology for nutritional deficiencies would allow a more meaningful comparison among previously published and future studies, but will definitely provide leverage for the overall field of bariatric surgery research. In the context of the present paper, we propose to standardise the definition of iron deficiency based on a combination of iron status markers. These markers should include at least ferritin concentration, which is relevant in the absence of underlying inflammation, and TSAT, which provides more reliable information in the presence of underlying information. However, future studies in bariatric patients are needed to assess the most optimal cut-off of values for ferritin concentration and TSAT to assess the prevalence of iron deficiency and to assess how to prevent and treat iron deficiency after bariatric surgery. Therefore, we advocate for more nutritional research in order to provide strong evidence-based guidelines after bariatric surgery.
Acknowledgements
The authors are grateful to the organisers of the 2017 international nutrition student research championships for the invitation to present this paper.
Financial Support
None.
Conflicts of Interest
None.
Authorship
N. S. and C. M. wrote the manuscript; B. VdS., A. M. and M. L. read and approved the final manuscript from a clinical point of view, while T. G. and P. A. read and approved the final manuscript from a more mechanical point of view. N. S. had primary responsibility for final content.