- IV
intravenous
- MEWS
medical early warning score
- OD
oedematous
Those working in the field of clinical nutrition have long recognised the sick oedematous (OD) post-operative patient referred for intravenous (IV) nutrition because of prolonged post-operative ileus. Many of these patients have marked peripheral or generalised oedema and hence are also likely to have intestinal wall oedema contributing to their prolonged post-operative gut dysfunction.
The UK Reference Nutrient Intake for sodium (Na) suitable for maintenance in normal adults is 70 mmol/24 h, which should be accompanied by about 1·5–2·5 litres (25–35 ml/kg/24 h) of water(Reference Itobi, Stroud and Elia3). Fluid and electrolyte homeostasis is maintained by the action of osmoreceptors and appropriate changes in vasopressin secretion affecting urinary concentration and free water clearance.
In the presence of salt depletion, the renin–angiotensin–aldosterone system is activated. The response of the body to low circulating volume and/or Na depletion is rapid and effective, while the converse is not true, perhaps because the excess salt availability that can with iatrogenic administration of saline, never occurred naturally in our evolutionary history. Even normal healthy subjects are therefore slow to excrete an excess Na load(Reference Powell-Tuck, Gosling and Lobo1, Reference Reid, Lobo and Williams4, Reference Williams, Hildebrand and McCormick5).
For surgical patients, it is even more difficult to excrete a salt and water load and to maintain normal serum osmolarity for reasons including the following:
1. The stress response to the injury or surgery leads to oliguria mediated by vasopressin, catecholamines and the renin–angiotensin–aldosterone system. Water and salt are therefore retained even in the presence of overload.
2. Following surgery, even when the serum osmolarity is reduced by administration of hypotonic fluid, the ability to excrete free water is limited(Reference Allison6) because the capacity of the kidney to dilute, as well as to concentrate the urine, is impaired. Excess free water infusion therefore risks dilutional hyponatraemia.
3. If saline is given, chloride (Cl) overload accompanies Na overload, with hyperchloraemia causing acidosis, renal vasoconstriction and further reductions in glomerular filtration rate and hence additional restriction in the ability to excrete Na and water.
4. Potassium (K) depletion, due to both the renin–angiotensin–aldosterone system activity and cellular loss of K which accompanies protein catabolism, also reduces the ability to excrete a Na load.
5. Metabolic products of catabolic breakdown produce a high renal solute load with consequent additional compromise to the ability to clear Na.
In recent years, the concepts outlined above led to concerns that the traditional peri-operative fluid management of 1 litre 0·9% saline and 2 litres 5% dextrose daily for most surgical patients provides too much fluid, Na and Cl and several randomised controlled trials have demonstrated reductions in post-operative complications and the length of hospital stay if routine post-operative daily fluid regimens are restricted to approximately 0·5 litre 0·9% saline and 1·5 litres 5% dextrose(Reference Lobo, Bostock and Neal7–Reference MacKay, Fearon and McConnachie9). Consequently, this led to the development of consensus UK National Guidelines on Post-Operative Intravenous Fluid Therapy for Adult Surgical Patients(Reference Powell-Tuck, Gosling and Lobo1) which was published in November 2008 recommending changes to the post-operative fluid management summarised below:
1. The details of fluids administered must be clearly recorded and easily accessible.
2. When patients leave the theatre, their volume status should be assessed. The volume and type of fluids given peri-operatively should be reviewed and compared with fluid losses in the theatre, including urine and insensible losses.
3. In patients who are euvolaemic and haemodynamically stable, a return to oral fluid administration should be achieved as soon as possible.
4. In patients requiring continuing IV maintenance fluids, these should be low in Na and of low-enough volume until the patients have returned their Na and fluid balance over the peri-operative period to zero. When this has been achieved, the IV fluid volume and content should be those required for the daily maintenance and replacement of any ongoing additional losses.
5. In patients who are OD, hypovolaemia if present must be treated followed by a gradual persistent negative Na and water balance based on urine Na concentration or excretion.
These recommendations fitted well with previous observations from our own research group describing the relationships between post-operative fluid management, the incidence of oedema and clinical outcome(Reference Itobi, Stroud and Elia2). Our data showed that although OD post-operative patients received approximately the same amount of IV fluid as non-OD patients, they were unable to excrete fluid efficiently. The OD patients then had increased lengths of stay, complication rates and greater requirements for nutritional support. We therefore decided to introduce most of the new UK consensus recommendations and present here the results of our efforts to improve the peri-operative fluid prescribed and the impact of these changes on clinical outcomes.
Interventions
A peri-operative fluid prescribing working group was set up in October 2007 and met six times over the following 13 months. The group comprised members of the Nutrition Support Team, Surgery, Anaesthetics, Intensive Care, Pharmacy, Medical School and Nursing Teams. Several initiatives to improve appropriate peri-operative fluid prescription were proposed.
Educational initiatives
Formal 30 min-structured educational sessions outlining the key evidence for rational fluid prescription were delivered to consultant surgeons and anaesthetists as well as junior medical staff. We felt it was essential to carry out this ‘top-down’ and ‘bottom-up’ approach as without consultant support, changes to traditional fluid prescribing would not take place. In addition, the junior staff actually writing the fluid prescriptions needed to be aware of the problems surrounding peri-operative fluid prescribing in order to effect change.
The aim of each educational session was to ensure that attendees should understand why fluid balance was important and when and when not to use 0·9% saline. They should also be able to assess a patient's hydration status and incorporate biochemistry results into effective fluid prescribing rather than simply repeating the previous fluid prescription on a chart handed to them. They were also made aware of the importance of trying whenever possible to prescribe fluid for all their own patients rather than leaving the job for a harassed junior doctor covering the wards at night.
The education sessions were repeated on several occasions and a formal teaching session on fluid prescribing was incorporated into the surgical junior doctor induction. Repetition and induction teaching were important due to the high turnover rates of junior staff.
Guideline development
An important function of the working group was to develop formal local guidelines on peri-operative fluid prescribing which were ratified by the drug and therapeutic prescribing committee in October 2008. The guidance was posted on the hospital intranet and their presence was widely advertised. However, since the assessment of hydration status and appropriate prescription of fluid is complex, the comprehensive guidance covering most clinical situations was quite long. Clearly this posed a problem, as it was not likely that busy junior doctors would read through lengthy guidelines at the point of prescribing. We therefore produced a pocket-sized portable laminated version of the guidance to give to all junior doctors at the end of fluid education sessions.
Our local guidelines are produced in full in the Appendix for the benefit of readers trying to improve fluid prescription practice in their hospital.
Changes to drug charts
The working group also wanted to ensure that body weight-based calculations of the likely maintenance requirements for fluid and electrolytes in individual patients was entered at the head of all fluid prescription charts beside some summary information on the fluid and electrolyte content of some commonly prescribed fluids (Fig. 1).

Fig. 1. Proposed changes to fluid prescription charts.
However, this proposal met with difficulties because of concerns that the guidance might mistakenly be applied to neuro-surgical patients for whom generous normal saline provision is often advocated, as well as to paediatric patients who have very different maintenance requirements for fluid and electrolytes per kg body weight. We were therefore unable to instigate these proposals.
Changes to medical early warning score
Our hospital uses the medical early warning score (MEWS) system which is designed to ensure early recognition of patients who are deteriorating in order to facilitate prompt medical attention and thus reduce morbidity and mortality. The system scores common clinical observations such as pulse, blood pressure, respiratory rate and urine output, with scores added together to produce a total that may trigger a call for urgent medical review. The doctor who sees the patient is then obliged to take appropriate action and since a low urine output scores highly in the MEWS system, a MEWS warning can often precipitate a ‘fluid challenge’ with either 0·9% saline or gelofusine.
We believed that a substantial proportion of our surgical patients triggered MEWS simply because of low post-operative urine outputs due to the ‘normal’ physiological, fluid retentive process that follows any injury, coupled with normal overnight reductions in urine volumes due to diurnal variation. These patients can then end up receiving inappropriate fluid challenges followed by additional, ongoing fluid prescription. Once again, however, although our working group aimed to address this problem, we could not identify a simple, robust and reliable alternative to the existing MEWS criteria which did not potentially miss truly hypovolaemic patients. The MEWS criteria therefore remained unchanged and instead the issue was particularly highlighted in the doctor education sessions with emphasis on the need to fully assess likely circulating volume in oliguric patients before initiating any fluid challenge as well as the need to carefully observe the effects of any fluid challenge before continuing with what could be inappropriate efforts to expand the circulating volume.
Routine use of 0·9% saline as the diluent
The working group found that 0·9% saline was used as the diluent for most drugs that needed diluting for IV administration. This was therefore changed to 5% dextrose wherever possible in order to reduce unnecessary Na and Cl administration which can add up to very significant quantities with some IV drugs in some patients.
Assessment methods and results
Ideally, the success of the interventions outlined above would have been determined by studies on fluid balance, body weight and measurements of body water(Reference Westerterp10) in large numbers of individual patients within a randomised controlled trial. However, the nature of our interventions was such (particularly those on staff education) that this could only have been achieved using a cluster-randomised trial model, with the measures introduced on some whole surgical wards while other whole wards maintained their previous prescription and educational standards. Inevitably, this would have then introduced multiple additional factors affecting clinical outcomes and the length of stay especially effects from different surgical, anaesthetic and nursing teams working on different wards in different hospitals. Simple power calculations suggested that such a trial would have required at least 84 surgical wards, across multiple hospital sites at a cost of several million pounds, and we therefore decided to undertake pragmatic studies comparing historical data from our previous 2002 study on fluid prescription, fluid balance, clinical oedema status and clinical markers of outcome in post-operative patients(Reference Itobi, Stroud and Elia2) with similar data from two prospective audits carried out in 2008 and 2009 after the implementation of the new measures. Cost and the need to make historical comparisons then precluded actually measuring body water in our pragmatic study with techniques such as deuterium-labelled water(Reference Westerterp10).
Patient demographics
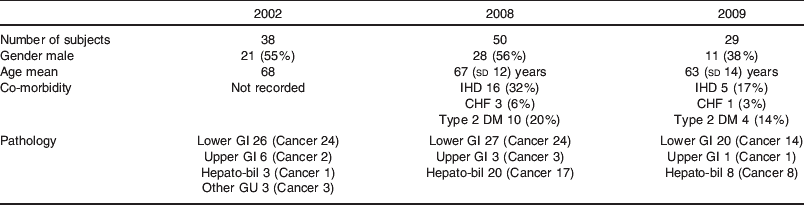
A comparison of the demographics of patients in Fluid Study 2002(Reference Itobi, Stroud and Elia2) and patients in the Prospective Audits 2008 and 2009 is shown in Table 1.
Table 1. Demographics of patients in Fluid Study 2002(Reference Itobi, Stroud and Elia2)v. Prospective Audits 2008 and 2009
Historical data and prospective audits
Historical data on fluid prescription and clinical outcomes in elective post-operative patients from 2002(Reference Itobi, Stroud and Elia2) were compared with two prospective audits carried out in 2008 and 2009. Detailed records for each patient in the prospective audits were kept. Information on their type of surgery, length of stay, complication rates, clinical observations and biochemistry were recorded. In addition, details on the type and quantity of fluid administered intra- and post-operatively for 5 d as well as all fluid losses were recorded on a daily basis. This enabled accurate fluid balance and Na administration to be calculated. All laparoscopic procedures were excluded. Patient demographics are presented in Table 1, while results data are presented in Tables 2 and 3 and Fig. 2.

Fig. 2. Net fluid balance post-operative (days 0–5), P=0·034, P (trend)=0·01.
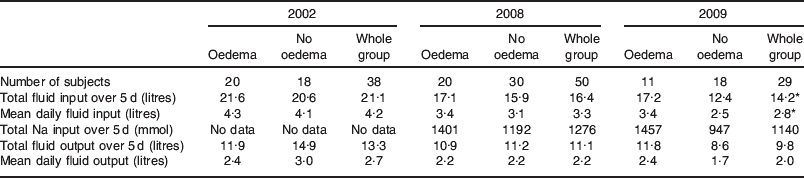
Table 2. Fluid input/output and Na administration: breakdown by the presence of oedema
* P=0·030 compared with 2002.
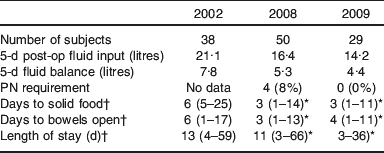
Table 3. Clinical outcomes over the three study years
* P<0·05 compared with 2002.
† Mean values and range.
Table 2 shows the fluid input and output as well as Na inputs from patients in 2002, 2008 and 2009 broken down by the presence of oedema. Overall, there is a trend where patients receive less fluid over progressive years, as seen in the fluid balance data (Fig. 2).
It can be seen that in 2002 and 2008, both OD and non-OD patients received approximately the same amount of fluid, while in 2009 the non-OD patients appeared to receive less fluid than their OD counterparts. While it is unfortunate that no Na data exist for the 2002 patients, the audit data from 2008 and 2009 show that non-OD patients received less Na than the OD patients.
We postulate that these data show that while a proportion of vulnerable patients develop oedema in their post-operative course, some of them can be protected from this occurrence by restricting their fluid and Na input.
Fluid balance over the first 5 post-operative days fell progressively over the three study periods as outlined in Fig. 2.
Pharmacy data
In order to corroborate our audit findings, we accessed pharmacy records to survey the quantity and type of fluid supplied to surgical wards. The data only included 1000 and 500 ml bags of fluid and not colloid and the records took no account of case numbers or case mix of patients on the surgical wards. Nevertheless, we believe that these records of the relative proportions of different fluids supplied to the general surgical wards during the period in question (March 2007–March 2009) are very likely to reflect changes in prescribing habits triggered by the interventions made.
Figure 3 shows that the proportion of 0·9% saline supplied to the wards prior to the inception of the fluid prescribing working group was fairly stable at just under 70% of all fluids supplied while 4%/0·18% dextrose–saline made up <10% of the total and Hartmann's solution <15%. After the introduction of the measures to reduce unnecessary 0·9% saline prescription, the percentage of 0·9% saline supplied fell gradually to approximately 35% of all fluid supplied, half of the original proportion, while 4%/0·18% dextrose–saline overtook 0·9% saline as the most common fluid prescribed at about 40% of all fluid supplied. There was also an increase in the use of Hartmann's solution to over 20%.

Fig. 3. The proportion of different, commonly prescribed fluids supplied to surgical wards. IVI, intravenous infusion.
Presence of oedema
The presence of oedema was noted in all subjects and was further broken down by severity using a simple validated scoring system (1=no oedema, 2=mild oedema (just detectable), 3=moderate oedema (significant but localised) and 4=severe oedema (extensive)). The incidence of any oedema declined from 52% of all patients in 2002 to 40% and 38%, respectively in 2008 and 2009. Of more interest is the fact that those with significant oedema (scores of 3 or more) declined from 25% in 2002 to 4% and 14% in 2008 and 2009, respectively.
Clinical outcomes
Clinical outcomes over the 3 study years are shown in Table 3. It can be seen that in line with fluid input over the 5 post-operative days decreasing over the study periods 2002, 2008 to 2009 from 21·1 to 16·4 to 14·2 litres, patients’ gut recovery after major abdominal surgery, as indicated by days to passing flatus and solid food, improved from 6 d to 3 d. Furthermore, the length of stay decreased correspondingly over this time from 13 to 11 d and then to 10 d.
Discussion
Two key interventions were made by our fluid prescribing working group with the aim of better aligning post-operative fluid prescription in our hospital with the new consensual guidelines(Reference Powell-Tuck, Gosling and Lobo1). Firstly, repeated education sessions to both consultants and junior doctors enabled prescribers to know why fluid prescription was important, while secondly, the publication of local fluid prescribing guidelines on both the hospital intranet and as easily usable, portable pocket formats enabled prescribers to know how to prescribe effectively. Our study then determined whether these interventions had any effect.
We freely acknowledge the shortcomings of the overall approaches used in this pragmatic study and ideally, as stated above, would have undertaken a cluster-randomised controlled trial of our interventions. Nevertheless, this would have entailed immense and costly logistical challenges and several years of work and we believe that our alternative, pragmatic, before-and-after, bed-side/documentation review study has produced data that show clear changes in fluid prescribing practices in our hospital between 2002 and 2008/9. These changes included reduced levels of overall fluid prescription in terms of volume and a move away from 0·9% saline usage with concomitant increase in the use of 4%/0·18% dextrose saline and Hartmann's solution.
The increased use of Hartmann's solution was perhaps more than we had expected, since the new local guidance had emphasised that dextrose saline with added K was a good maintenance fluid as long as it was not used in high volume to replace abnormal rates of fluid loss. However, the use of Hartmann's solution instead of 0·9% saline was included in our guidance and this possibility had been given greater emphasis on the original UK consensus guidance. Furthermore, the increased prescription of Hartmann's solution we witnessed was primarily driven by a change in the prescribing habits of anaesthetists who tended to prescribe post-operative fluids for the first 24 h, and we understand that they as a group were also hearing about the need to reduce saline prescription from other non-local sources which tended to emphasise Hartmann's solution as a good alternative.
The changes that we documented not only demonstrated success in altering fluid prescription in our hospital towards the recommendations set out in the new UK guidance, but also demonstrated significant improvements in our other outcome measures including reductions in the positivity of fluid balance, fluid overload manifest as oedema, days to return of bowel function and the overall length of hospital stays. Clearly, not all these outcomes are entirely objective and accurate and, in particular, we recognise that fluid chart recording is notoriously problematic. Nevertheless, steps were taken to reduce such problems with ward nurses made aware of the audit and asked to be assiduous in their fluid recordings, and data were collected prospectively wherever possible with careful checks of both fluid prescription charts and fluid intake/output records. Furthermore, any inaccuracies in the fluid data recording should have been randomly spread among all patients, with no systematic bias.
It is, of course, possible that the improvements in clinical outcome documented in our study were reflections of other changes in patient care that took place between 2002 and 2009 and, in particular, this period saw the adoption of an enhanced recovery programme for surgical patients as well as our changes in recommendations for fluid prescription. However, since only non-laparoscopic patients requiring major surgery were included in our study, our findings cannot be accounted for by the enhanced programme, and we can think of no other changes in practice that are likely to have influenced our findings.
Conclusions
With sustained effort across different specialities, it is possible to improve knowledge of both why and how to prescribe fluids effectively and this can lead to a change in practice with reductions in both the overall volume of post-operative fluid prescription and in the prescription of normal saline. Such changes, in line with the recently published UK guidance(Reference Powell-Tuck, Gosling and Lobo1), appear to yield considerable clinical benefit as judged by our pragmatic study, and these benefits are in line with those previously reported from formal clinical trials(Reference Itobi, Stroud and Elia3, Reference Reid, Lobo and Williams4). Our data underline the need for continuous audit of clinical practice with appropriate analysis to determine the likely impacts of change and show that relatively simple, low-cost, pragmatic methodologies can be used to assess changes in clinical practice.
Acknowledgements
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. The authors declare no conflicts of interest. A. N. D. S. designed audit, collected audit data 2008 and 2009, analysed data and prepared the manuscript; T. S. collected audit data 2008; E. I. designed, collected the data and analysed the Fluid study 2002(Reference Itobi, Stroud and Elia2); P. A. analysed the pharmacy records; M. A.-H. collected the audit data 2008; S. A. W. analysed the data and prepared the manuscript and M. A. S. designed the audit, was Chair of Fluid Working Group, analysed the data and prepared manuscript.
Appendix
Guidance on prescribing peri-operative IV fluids and electrolytes for adult Southampton University Hospitals NHS Trust patients (excluding neurosurgical patients).
What is your aim?
1. Before prescribing IV fluids, assess whether your patient really needs them and be clear whether your aim is for volume replacement/resuscitation or maintenance.
2. In general, oral or enteral tube delivery is safer than IV and less likely to cause salt and fluid overload.
3. Take into account the current hydration status (clinical, biochemical and fluid balance assessment) and current+‘likely-near-future’ oral intake as well as any excess losses.
4. If IV fluid is needed, the amount and type prescribed should meet specific fluid and electrolyte goals since both too little and too much can do harm.
5. After starting, monitor the fluid/electrolyte status both clinically and biochemically (initially daily) and adjust IV provision if oral intake or enteral/parenteral nutrition support is started.
6. Daily maintenance requirements for an adult NOT eating or drinking are shown in Table A.1.
7. The water and electrolyte content of commonly available IV fluids are shown in Table A.2.
Table A.1. Normal daily maintenance needs for water, Na, K and Cl in adultsFootnote *
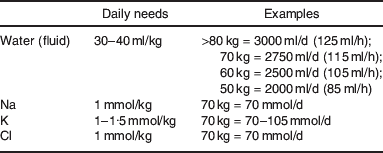
* Young children (especially infants and neonates) have very different fluid and electrolyte requirements, and neurosurgical patients may need large quantities of saline to reduce risk of cerebral oedema.
Table A.2. Electrolyte content of commonly available IV Fluids

Generally avoid ‘normal saline’ (154 mmol Na/l) unless there is a specific need to replace saline loss (e.g. high gastro-intestinal losses). This is because surgery triggers Na retention mechanisms and excess Na and Cl cause oedema, increased post-operative complications, delayed return of gastro-intestinal function and hyper-chloraemic acidosis.
Although dextrose and dextrose saline are good maintenance fluids, giving too much can cause dilutional hyponatraemia. However, many hyponatraemic patients have water excess rather than Na depletion (seek senior advice in patients with low plasma Na).
Remember that many patients receive or have received considerable additional Na loads from peri- or intra-operative colloid and other fluids, and some of their drugs e.g. many IV antibiotics, are made up in NaCl as a diluent.
Pre-operative fluids
1. The aim of the pre-operative fluid is to avoid dehydration which is uncomfortable and leads to risks such as renal impairment and hypotension with anaesthetic induction.
2. Since most patients can drink until 2 h before surgery, IV cannulae and fluids are only needed for those dehydrated or at particular risk of dehydration (e.g. from vomiting/nasogastric drainage, obstruction, diarrhoea, bowel preparation etc.); those with specific co-morbidities such as insulin dependent diabetes mellitus, electrolyte abnormalities, renal impairment and obstructive jaundice; or those needing other IV drugs.
3. Your aim is usually to provide maintenance fluid, Na and K as indicated in the tables above although sometimes you will need to treat dehydration, correct biochemical problems or match high GI losses (see below). Normal saline should not generally be used since needs are often best met using 2 to 3 litres of dextrose–saline (4% and 0·18%) with 2% KCl added to each litre (each litre then contains 1000 ml water, 30 mmol Na, 27 mmol K and 57 mmol Cl). Large volumes of dextrose-saline e.g. to correct hypovolaemia, should not be used since this can cause hyponatraemia. Extra care and senior advice is needed for patients with cardiac, hepatic or renal impairment.
Intra-operative and early post-operative fluids
All fluids given intra-operatively and for 24 h post-operatively are usually prescribed by the anaesthetists who often choose Hartmann's solution, colloid and blood to ensure the best possible post-operative fluid and electrolyte status. These prescriptions should not be altered unless there is an unexpected change in clinical status in which case senior review is often required.
Later post-operative ‘routine’ maintenance
1. IV fluids are often needed for a few days following operations, since many patients cannot eat and drink due to nausea, post-operative ileus or potentially vulnerable anastomoses.
2. Give maintenance water, Na and K as indicated in Table A.1 using the same approach as suggested in the ‘Pre-operative fluids’ section (see above) unless there is a specific need to treat dehydration, correct current biochemical problems or match high GI losses (see below).
Post-operative patients with excess gastro-intestinal losses
1. Patients with high losses from NG tubes, drains, high stomas, fistulas and diarrhoea will lose both fluid and lots of Na (e.g. about 60–100 mmol Na/l for upper GI fluid losses). They therefore need Hartmann's solution or 0·9% NaCl in addition to the normal maintenance fluids outlined above.
2. The prescription of the additional fluids should only match the extra GI loss (i.e. do not account for urinary or insensible loss when calculating these additional needs since these are covered by the maintenance component of your prescription).
3. In patients with normal renal function who are not taking Na clearing diuretics, a spot check showing a urinary Na >30 mmol confirms adequate Na provision.
4. Additional, i.e. above maintenance, K and/or magnesium (Mg) prescription may also be needed.
Fluid resuscitation for hypovolaemia
1. Poor urine output alone is often not caused by hypovolaemia but by normal Na and water retention mechanism after surgery and/or normal circadian rhythms. Fluid challenges should therefore be restricted to patients with other factors suggesting intravascular fluid depletion (see later).
2. Circulatory hypovolaemia is usually a consequence of dehydration, blood loss or sepsis. For simple, non-urgent correction of dehydration, use 5% dextrose or dextrose-saline (4% and 0·18%), but beware of hyponatraemia if large volumes are needed.
3. For urgent correction of suspected intravascular fluid depletion, use colloid and/or Hartmann's ‘fluid challenges’ following the algorithm in Fig. A.1, monitoring CVP unless the JVP can be clearly seen (N.B. may need patient to lie flat).
4. For urgent correction of blood loss, use blood guided by Hb levels with colloid or Hartmann's solution if hypovolaemia still present when adequate Hb is attained (N.B. with acute blood loss Hb may be relatively normal until there has been time for dilution).
5. For urgent correction of hypovolaemia in sepsis, use colloid or Hartmann's solution, but note that severely septic patients with circulatory collapse often need inotropic support both to reduce vasodilatation and peripheral extravasation of fluid, and to maintain cardiac output.

Fig. A.1. Southampton University Hospital Fluid Challenge algorithm. CVP, central venous pressure; JVP, jugular venous pressure; LV, left ventricle; BP, blood pressure.











