Maternal nutritional status during pregnancy may affect the epigenetic programming of offspring, including effects on DNA methylation patterns, and so lead to phenotypic consequences in adulthood. It is hypothesised that maternal Zn nutrition may affect the DNA methylation status of the offspring through effects on the expression of DNA methyltransferase (DNMT) enzymes. The present study investigated the effects of feeding a Zn-varied diet to pregnant mice on global DNA methylation in offspring and measured the effect of extracellular Zn concentration on the expression of DNMT in a cell-line model.
Pregnant mice were fed a Zn-restricted (15 μg/g), Zn-adequate (50 μg/g) or Zn-supplemented (150 μg/g) diet for the first 17 d of pregnancy. Mice were killed, fetal livers harvested and DNA extracted. Global DNA methylation was measured by the luminometric methylation assay (LUMA) assay using a pyrosequencer(Reference Karimi, Johansson and Stach1).
Significantly higher levels of methylation were observed in the DNA of mice fed both Zn-restricted and Zn-supplemented diets compared with mice fed the Zn-adequate diet (Figure).

Figure. Global methylation of fetal liver DNA measured by LUMA. Relative methylation levels are expressed as a ratio of the signal recorded by end-filling following digestion with HpaII/MspI, normalised to ECoR I, and shown as means with their standard errors represented by vertical bars for twelve mice for the Zn-restricted and Zn-adequate groups and nine mice for the Zn-supplemented group. Each analysis was run in duplicate. ***P<0.001 (one-way ANOVA, followed by Bonferroni's multiple comparisons test).
To investigate the effect of extracellular Zn on the expression of DNMT at the mRNA level, parallel Zn-supplementation studies were carried out in a human SW480 cell-line model. Cells were treated with either basal medium or medium supplemented with 50 μm- or 100 μm-ZnCl2 for 96 h. Total RNA was extracted and semi-quantitative RT–PCR analysis of DNMT1 and DNMT3a was carried out. Cells showed a significant increase in DNMT1 and DNMT3a mRNA expression at 100 μm-Zn compared with basal medium (Table).
Table. Analysis of DNMT mRNA levels expressed relative to glyceraldehyde 3-phosphate dehydrogenase mRNA levels in SW480 cells after 96 h exposure to different concentrations of Zn (n 6 for each treatment)†
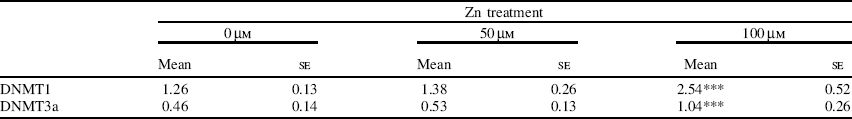
Mean values were significantly different from those for untreated cells (one-way ANOVA with least significant difference multiple comparisons test): ***P<0.001. †Values were obtained by densitometric band intensities from ethidium bromide-stained agarose gels.
The findings indicate the potential for Zn nutrition during pregnancy to influence global DNA-methylation patterns in the offspring. Previous analysis of placental RNA samples from the same study revealed reduced levels of transcripts for a number of Zn transporters in mice fed the Zn-restricted and Zn-supplemented diets compared with mice fed the Zn-adequate diet(Reference Helston, Phillips and McKay2). The current findings indicate that site-specific DNA methylation should be explored as a potential mechanism underlying these effects. The observed increase in DNMT mRNA at an increased concentration of extracellular Zn provides evidence in support of the idea that effects of Zn on DNA methylation may be mediated through Zn-dependent changes in the expression of these enzymes.




