Data from clinical trials, controlled metabolic interventions and prospective cohort studies indicate that the substitution of SFA and trans fatty acids (TFA) for PUFA lowers mortality and markers of CVD risk( Reference Hooper, Summerbell and Thompson 1 – Reference Skeaff and Miller 5 ). Most public health policies in developed countries recommend population wide decreases in the consumption of SFA and TFA and an increase in PUFA intake to lower the incidence of CVD and metabolic diseases( 6 – 8 ). Despite the establishment of nutritional guidelines, dietary surveys indicate that the intakes of SFA typically exceed recommended levels, while the consumption of PUFA, specifically n-3 PUFA is often below the optimal range( Reference Bates, Lennox and Prentice 9 – Reference Eilander, Harika and Zock 12 ). The majority of PUFA in the human diet originates from plant oils and vegetable fats containing relatively high proportions of linoleic acid (18 : 2 n-6) and linolenic acid (18 : 2 n-3), while intakes of the long chain n-3 PUFA, EPA (20 : 5 n-3) and DHA (22 : 6 n-3) contained in oily fish fall short of a recommended target of 450 mg/d( Reference Givens 13 ).
In most developed countries, meat and meat products are a significant source of fat and SFA in the human diet, but also contribute to 20 : 5 n-3, docosapentaenoic acid (22 : 5 n-3) and 22 : 6 n-3 consumption( Reference Bates, Lennox and Prentice 9 – Reference Givens 13 ). Ruminant-derived meat and meat products are also a source of TFA in the human diet( Reference Scollan, Hocquette and Nuernberg 14 – Reference Bessa, Alves and Santos-Silva 17 ). Altering the fat content and fatty acid (FA) composition of meat and meat products offers the opportunity to realign the consumption of FA in human populations closer to Public Health guidelines for lowering the incidence of non-communicable diseases without requiring substantive changes in consumer eating habits. Global meat consumption is projected to increase within the next 30 years( Reference Clonan, Roberts and Holdsworth 18 ), highlighting that the potential benefits and impact from altering the fat content and FA composition of poultry, pork, beef and lamb will become increasingly important. The present paper focuses on approaches to improving the lipid composition of meat. The impact on aspects of meat quality including colour, shelf life and sensory were recently reviewed( Reference Scollan, Dannenberger and Nuernberg 19 ) and hence not considered in the present manuscript.
Lipid in meat from monogastric and ruminant animals
Lipid content of meat varies depending on muscle and tissue type, animal species and production system that affect nutritional, sensory and technological properties and overall quality( Reference Scollan, Hocquette and Nuernberg 14 , Reference Wood, Enser and Fisher 20 ). Furthermore, FA composition determines the physical and textural properties of adipose and the oxidative stability of muscle, which affects flavour, juiciness, tenderness, muscle colour and overall liking. Fat in meat is deposited in intramuscular, intermuscular and subcutaneous adipose stores mainly in the form of glycerol esters, cholesterol, phospholipids and FA esters. Intramuscular fat (IMF) content of chicken, pork, beef and lamb typically varies between 10–25, 15–40, 20–50 and 30–80 g/kg, respectively( Reference Scollan, Hocquette and Nuernberg 14 , Reference Sinclair 15 , Reference Wood, Enser and Fisher 20 , Reference Rymer and Givens 21 ). For chicken, the lipid content of dark meat and light meat averages 28 and 11 g/kg, respectively( Reference Rymer and Givens 21 – Reference Mirshekar, Boldaji and Dastar 26 ). The IMF of chicken and pork contains 260–350, 290–460 and up to 200 g/100 g, as SFA, MUFA and PUFA, respectively( Reference Rymer and Givens 21 – Reference Gjerlaug-Enger, Haug and Gaarder 33 ). In beef and lamb, IMF contains 450–480 and 350–450 g/100 g of SFA and MUFA, respectively and up to 50 g/100 g as PUFA, respectively ( Reference Bessa, Alves and Santos-Silva 17 , Reference Clonan, Roberts and Holdsworth 18 ). The ratio of PUFA:SFA in IMF of beef or lamb is typically low at about 0·1–0·2 except for very lean animals (<10 g/kg IMF) or animals fed rumen protected lipid supplements where this ratio can be as high as 0·5–0·7( Reference Scollan, Hocquette and Nuernberg 14 , Reference Sinclair 15 ). The ratio of n-6:n-3 PUFA in ruminant meat (abundance of α-linolenic acid (18 : 3 n-3), and to a lesser extent 22 : 5 n-3 and 22 : 6 n-3, relative to 18 : 2 n-6 and arachidonic acid (20 : 4 n-6)) from pasture or diets based on grass or forage legume silages is generally <3·0, but this ratio can exceed 5·5 in animals fed high amounts of cereal grains( Reference Scollan, Hocquette and Nuernberg 14 , Reference Sinclair 15 ).
Lipid in beef, lamb and other ruminant meat products also contain isomers of conjugated linoleic acid (CLA) and TFA. Depending on muscle type, production system and breed the proportions of total CLA and TFA in retail beef vary between 0·34–0·82 and 2·97–5·63 g/100 g total FA, respectively, corresponding to between 9·8–98 and 70–586 mg/100 g muscle( Reference Kraft, Kramer and Schoene 34 – Reference Aldai, Dugan and Kramer 36 ). Measurements for retail lamb are limited, but a recent report indicated that the proportions of total CLA and TFA accounted for 0·59–1·44 and 6·41–12·0 g/100 g FA( Reference Bravo-Lamas, Barron and Kramer 37 ).
Nutritional approaches to enhance fatty acid composition
Diet is known to influence the FA composition of meat and meat products. Numerous investigations have examined the potential to: (i) lower the relative proportions of SFA, (ii) increase the overall PUFA:SFA ratio and (iii) enrich n-3 PUFA relative to n-6 PUFA in intramuscular lipid. In ruminants, increases in specific FA, including cis-9, trans-11 CLA have been targeted. Most studies have focused on including oilseeds, plant oils, fish oils, marine algae in the diet of pigs( Reference Nuernberg, Fischer and Nuernberg 27 – Reference Gjerlaug-Enger, Haug and Gaarder 33 ), poultry( Reference Cortinas, Villaverde and Galobart 22 , Reference Azcona, Garcia and Cossu 23 , Reference Kalogeropoulos, Chiou and Gavala 24 – Reference Mirshekar, Boldaji and Dastar 26 ), cattle( Reference Kim, Richardson and Gibson 38 – Reference Pouzo, Fanego and Santini 41 ) and sheep( Reference Annett, Carson and Fearon 42 – Reference Meale, Chaves and He 45 ) to alter meat FA composition and content. In ruminants, the use of lipid supplements protected from ruminal metabolism have also been investigated( Reference Scollan, Hocquette and Nuernberg 14 , Reference Sinclair 15 , Reference Gulati, Garg and Scott 46 , Reference Dunne, Rogalski and Childs 47 ). Both the processes of digestion and metabolism of absorbed lipid in the host animal have a major impact on the transfer efficiency of dietary FA into meat. In monogastric animals, the small intestine is the major site for the digestion of dietary lipid. Digestion involves the action of pancreatic lipase to hydrolyse TAG into 2-monoacylglycerol and free acids and the formation of micelles followed by absorption in the intestinal mucosa and transport of FA in the peripheral circulation for uptake by body tissues mediated by lipoprotein lipase( Reference Woods and Fearon 48 ). In pigs and poultry, dietary lipid remains largely intact before absorption and incorporation into tissue lipid, and therefore changes in dietary FA intake have a largely predictable influence on tissue lipid composition. Digestion of dietary lipid in ruminants is far more complicated due to the metabolic activity of the microbial community in the rumen-reticulum. As a result, meat from ruminant animals, such as beef and lamb, contains a more diverse range of FA that bear little resemblance to the composition and amount of FA supplied by the diet( Reference Vahmani, Mapiye and Prieto 49 ). Dietary unsaturated FA, PUFA in particular, have toxic effects on certain rumen microorganisms( Reference Maia, Chaudhary and Figueres 50 , Reference Zhang, Guo and Yuan 51 ). To alleviate the inhibitory effects on growth, the rumen microbiome has evolved to secrete proteins capable of hydrolysing ester bonds of esterified FA and decreasing the degree of unsaturation of the free FA released through reduction, isomerisation or hydration. Lipolysis and biohydrogenation result in extensive metabolism of dietary PUFA to saturated end-products limiting the escape of dietary PUFA from the rumen. However, biohydrogenation is incomplete, resulting in the formation of FA intermediates often containing one or more trans double bonds( Reference Shingfield, Bonnet and Scollan 16 , Reference Kim, Huws and Lee 52 ), which following absorption are used as substrates for tissue lipogenesis. Understanding the mechanisms responsible and microbiota and their associated enzymes capable of these reactions is central to future attempts to develop nutritional strategies for strategic and more predictable changes in the FA composition of ruminant meat.
Potential for reengineering ruminal lipid metabolism
Understanding of the role of rumen bacteria in biohydrogenation has traditionally been based on investigations with bacteria able to be cultured ex vivo. Culturable Butyrivibrio spp. capable of biohydrogenation have been the most widely studied. However, development of new molecular methods have enabled more informed investigations of rumen microbiome–lipidome interactions( Reference Kim, Huws and Lee 52 – Reference Privé, Newbold and Kaderbhai 55 ) based on experiments involving the use of dietary lipid supplements to alter ruminal lipid metabolism and application of next generation sequencing technologies to characterise the impact on the metataxonome. Changes in the ruminal bacterial taxa have highlighted that the communities involved in biohydrogenation are potentially much more diverse than implicated from historical studies with pure cultures( Reference Huws, Kim and Lee 54 – Reference Boeckaert, Vlaeminck and Fievez 56 ) that are now known to include Prevotella, Lachnospiraceae incertae sedis and unclassified Bacteroidales, Clostridiales, Succinovibrio, Roseburia and Ruminococcaceae, species identified as yet unculturable. Such findings highlight the challenges to developing targeted approaches for altering the biohydrogenation activity of the rumen microbiota.
Much less is known about the microbiology underpinning lipolysis of esterified lipid in the rumen. Few culturable isolates with known lipolytic activity have been identified, and historically only bacterial genera, namely Anaerovibrio lipolyticus and Butyrivibrio spp. have been shown to have lipolytic capacity, with A. lipolyticus being specific to TAG and Butyrivibrio being specific towards phospholipids. Nonetheless, the lipases possessed by these bacteria and others within the rumen microbiome have until recently remained relatively understudied. Recently, the creation of rumen bacterial fosmid-based metagenomic libraries, enabled twelve lipase/esterase genes and two phospholipases to be isolated, the sequences of which appear to originate from bacteria that cannot as yet be cultured ex vivo ( Reference Privé, Newbold and Kaderbhai 55 ). A draft genome of A. lipolyticus coupled with annotation and biochemical characterisation also allowed the characterisation of three identified lipases with activity against TAG( Reference Privé, Kaderbhai and Girdwood 57 ). This new knowledge will be invaluable to understanding the biological potential to alter the extent of lipolysis in the host ruminant in the future.
Both bacteria and protozoa leaving the rumen are also an important source of FA available for absorption by the host animal. Membrane lipids of rumen bacteria contain relatively high proportions of odd-chain and branched-chain FA. Rumen protozoa are relatively rich in MUFA, PUFA and isomers of CLA, possibly due to engulfment of chloroplasts that contain the majority of 18 : 3 n-3 in structural components of plant thylakoid membranes( Reference Hawke, Butlerand and Bailey 58 – Reference Huws, Kim and Kingston-Smith 60 ). Intra-protozoal chloroplast lipid metabolism may also facilitate the direct uptake of the major FA in chloroplasts (16 : 0, 18 : 2 n-6 and 18 : 3 n-3) into protozoal membranes. Furthermore, co-localisation of chloroplasts and engulfed bacteria within food vacuoles may also lead to intra-protozoal lipolysis and the biohydrogenation of PUFA in ingested chloroplasts, assuming that co-localised bacteria exhibit lipolytic and biohydrogenation activity. Such a mechanism may explain the rather high proportion of CLA isomers in the lipid of rumen protozoa. Nevertheless, increases in intra-protozoal chloroplast content do not appear to increase ruminal escape of PUFA( Reference Huws, Lee and Kingston-Smith 59 ). Zero grazing of growing steers was found to elevate intra-protozoal chloroplast content compared with a semi-synthetic diet based on straw and concentrates, but the amount of PUFA reaching the duodenum did not differ between dietary treatments as the protozoal flow from the rumen to the duodenum was low following zero grazing. It is hypothesised that perhaps the higher sugar content of grass and subsequent chemotaxis of protozoa towards sugars may enhance their likelihood of remaining within the rumen. Future investigations are required to establish whether it is possible to simultaneously increase the PUFA content of protozoa and increase outflow of PUFA-enriched protozoa from the rumen.
Dietary sources of PUFA
While forages are rarely used to support the nutritional requirements of monogastric animals, these represent important feed resources for ruminants. It is well established that diets based on fresh or conserved forages typically result in higher n-3 PUFA and lower n-6 PUFA content of lamb and beef compared with cereals( Reference Scollan, Hocquette and Nuernberg 14 , Reference Sinclair 15 , Reference Fisher, Enser and Richardson 61 – Reference Aldai, Dugan and Kramer 65 ). Even though forage has a relatively low lipid content, varying between 30 and 100 g/kg DM, it is a rich source of PUFA, particularly 18 : 3 n-3, which accounts for 50–75% of total forage FA content in grasses and forage legumes( Reference Hawke, Butlerand and Bailey 58 , Reference Dierking, Kallenbach and Roberts 66 ). Depending on production system, forages are often the primary source of FA in the ruminant diet( Reference Hawke, Butlerand and Bailey 58 , Reference Dewhurst, Scollan and Lee 67 ), which in addition to being relatively inexpensive and a sustainable feed resource, underpins an expanding market for ‘grass-fed’ or ‘grass-finished’ ruminant meat products with a lower total fat and increased n-3 PUFA content( Reference Daley, Abbott and Doyle 68 – Reference Howes, Bekhit and Burritt 70 ). Both environment and genetics influence FA biosynthesis and forage lipid content( Reference Morgan, Huws, Scollan, Golinski, Warda and Stypinski 69 , Reference Glasser, Doreau and Maxin 71 , Reference Hegarty, Yadav and Lee 72 ), highlighting the potential to select for grasses with a higher lipid content to increase PUFA intakes in the host ruminant. Several secondary metabolites in plants have been suggested to afford some protection of forage PUFA from lipolysis and biohydrogenation in the rumen. Many of these compounds are associated with a variety of ‘weed’ species common in species-rich pasture( Reference Morgan, Huws, Scollan, Golinski, Warda and Stypinski 69 ) that include condensed tannins( Reference Min, Barry and Attwood 73 , Reference Vasta, Makkar and Mele 74 ), saponins( Reference Shi, Arunasalam and Yeung 75 , Reference Wallace 76 ), catecholamines( Reference Lafontan, Berlan and Stich 77 ) and essential oils( Reference Wallace 76 ).
Oilseeds are rich in C18 unsaturated FA, but differ in relative abundance of oleic acid (cis-9 18 : 1), 18 : 2 n-6 and 18 : 3 n-3, which have been used to alter the FA composition of poultry meat and pork. Rapeseed is rich in cis-9 18 : 1, sunflower and safflower contain high proportions of 18 : 2 n-6, while linseed, flaxseed and camelina are common sources of 18 : 3 n-3. Use of dietary supplements of linseeds and linseed oil have been the most widely investigated for enriching n-3 PUFA in meat from chickens, pigs and ruminants. In addition to elevating 18 : 3 n-3 concentrations, the abundance of 20 : 5 n-3, 22 : 5 n-3 and 22 : 6 n-3 in intramuscular lipid is often increased( Reference Dewhurst, Shingfield and Lee 63 , Reference Dewhurst, Scollan and Lee 67 , Reference Barceló-Coblijn and Murphy 78 , Reference Scollan, Hocquette and Richardson 79 ) due to the elongation and desaturation of 18 : 3 n-3.
Fish oil and marine algae are the richest available sources of 20 : 5 n-3, 22 : 5 n-3 and 22 : 6 n-3 and have been used as dietary supplements to increase the long chain n-3 PUFA content of meat. In pigs and chickens the extent of long-chain n-3 PUFA enrichment is determined by the level of supplementation( Reference Cortinas, Villaverde and Galobart 22 , Reference Rymer, Gibbs and Givens 25 , Reference Meadus, Duff and Uttaro 30 ). Despite extensive metabolism of 20 : 5 n-3, 22 : 5 n-3 and 22 : 6 n-3 in the rumen( Reference Kim, Huws and Lee 52 , Reference Shingfield, Ahvenjarvi and Toivonen 80 , Reference Lee, Shingfield and Tweed 81 ), marine lipid supplements can be used to increase the long-chain n-3 PUFA content of beef and lamb( Reference Angulo, Hiller and Olivera 40 , Reference Annett, Carson and Fearon 42 , Reference Hopkins, Clayton and Lamb 44 , Reference Meale, Chaves and He 45 ). However, both fish oil and marine algae also inhibit the complete biohydrogenation of C16 and C18 unsaturated FA in the rumen causing numerous trans mono- and polyenoic intermediates to accumulate and a decrease in 18 : 0 formation( Reference Boeckaert, Vlaeminck and Fievez 56 , Reference Shingfield, Ahvenjarvi and Toivonen 80 – Reference Shingfield, Kairenius and Arölä 84 ), changes that lead to an increase in the TFA content of beef( Reference Angulo, Hiller and Olivera 40 ) or lamb( Reference Annett, Carson and Fearon 42 , Reference Hopkins, Clayton and Lamb 44 , Reference Meale, Chaves and He 45 ).
Specialised lipid supplements have been developed to be more resistant to lipolysis and biohydrogenation in the rumen to increase the amount of PUFA available for deposition, elongation and desaturation in muscle and adipose tissue. Protected lipid also minimise the adverse effects of lipid on ruminal digestion and can be used to increase the amount of fat in the earlier recommended ruminant diet levels (<60 g/kg diet DM). Various technologies have been developed in an attempt to protect plant or marine lipid sources from lipolysis and biohydrogenation in the rumen, that include encapsulating oils with a protein matrix and treating with formaldehyde; feeding lipid as calcium soaps or FA amides; physical processing of oilseeds (heating, grinding, cracking, bruising, rolling, extruding) or whole intact oilseeds( Reference Scollan, Dannenberger and Nuernberg 19 , Reference Gulati, Garg and Scott 46 , Reference Jenkins and Bridges 85 ). However, the potential to increase the outflow of PUFA from the rumen using these supplements is often rather limited, and known to vary depending on the method used and source of protected lipid( Reference Jenkins and Bridges 85 ). Inevitably, the use of protected supplements also increase the cost of ruminant meat production that would need to be recovered by a premium at retail( Reference Scollan, Dannenberger and Nuernberg 19 ).
Enrichment of PUFA in meat from monogastrics
Two main approaches have been used to increase the n-3 PUFA content of pork and chicken that include dietary supplements of linseed or flaxseed as a source of 18 : 3 n-3, and the use of fish oil or marine algae as a source of 20 : 5 n-3 and/or 22 : 6 n-3 and demonstrated the potential to enrich the n-3 PUFA content of intramuscular lipid in growing pigs and chickens (Tables 1 and 2, respectively). Dietary supplements of oilseeds can be used to increase in the 18 : 3 n-3 content of muscle in pigs ( Reference Nuernberg, Fischer and Nuernberg 27 – Reference Guillevic, Kouba and Mourot 29 , Reference Bertol, de Campos and Ludke 31 – Reference Gjerlaug-Enger, Haug and Gaarder 33 ). Even though cis-9 18 : 1 is the major FA in rapeseed and olive oil, these lipid can be used to elevate 18 : 3 n-3 in pork, but to a much lesser extent than linseed or flaxseed (Table 1). Enrichment of 18 : 3 n-3 in body tissues of growing pigs is determined by the amount of flaxseed in the diet( Reference Turner, Mapiye and Aalhus 32 ). Supplements of flaxseed (50 g/kg diet) resulted in a 5-fold increase in 18 : 3 n-3 content relative to the control diet, while higher inclusion rates (100 g flaxseed/kg diet) resulted in a 15-fold enrichment of 18 : 3 n-3 than the control. Dietary supplements of marine algae have also been shown to result in dose dependent increases in 22 : 6 n-3 content of bacon( Reference Meadus, Duff and Uttaro 30 ). Enrichment of 20 : 5 n-3, 22 : 5 n-3 and 22 : 6 n-3 was higher for pigs fed diets containing algae at 16 g/kg compared with 6 g/kg. The potential of using a mixture of linseeds and fish oil to alter the n-3 PUFA content of pork has also been investigated( Reference Haak, De Smet and Fremaut 28 ). Linseed supplementation increased 18 : 3 n-3 content and enriched 20 : 5 n-3, 22 : 5 n-3 and 22 : 6 n-3 due to elongation and desaturation of 18 : 3 n-3 in body tissues. Much higher increases in 20 : 5 n-3, 22 : 5 n-3 and 22 : 6 n-3 content were achieved in growing pigs fed diets containing fish oil with minimal effects on the proportion of 18 : 3 n-3 in total lipid.
Table 1. Effect of dietary lipid supplements on the PUFA content of pork (mg/100 g)
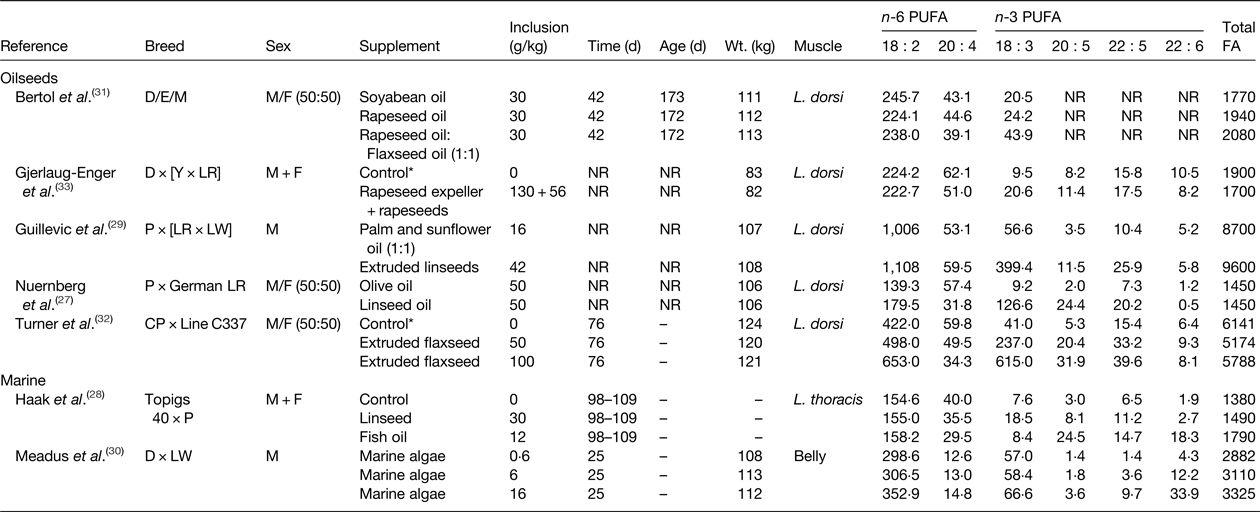
D, Duroc; E, Embapa; Y, Yorkshire; LR Landrace; P, Pietrain; LW, Large White; CP, Camborough Plus., M, Moura, NR, not reported.
* Control diet contained animal fat that was completely or partially replaced with test lipid supplements.
Table 2. Effect of diet on the fatty acid (FA) content of chicken breast (mg/100 g)
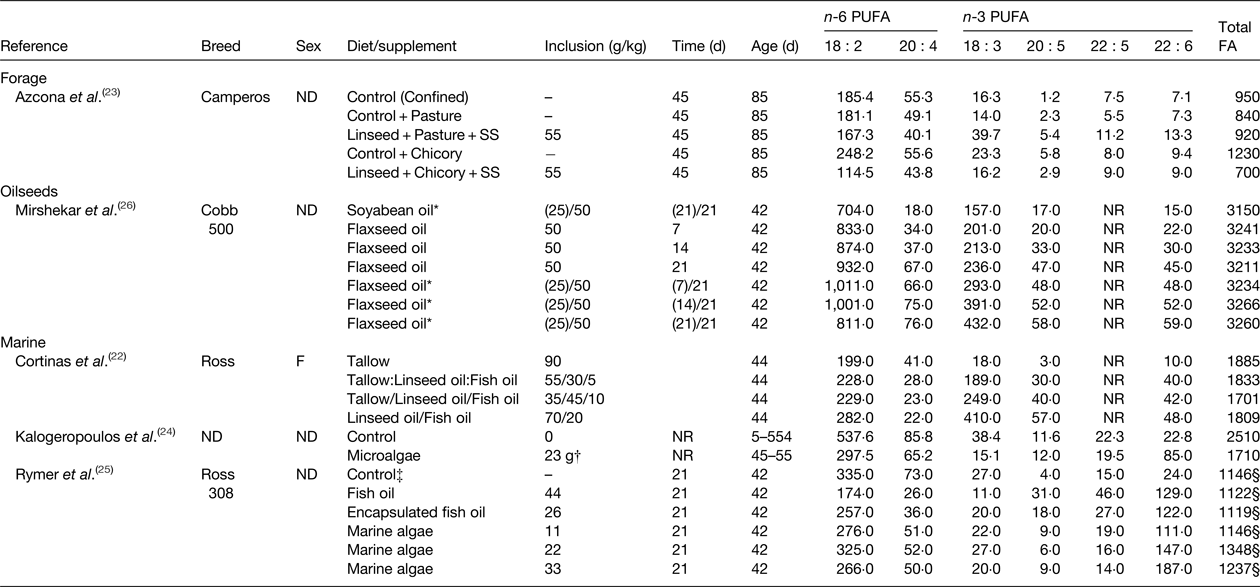
ND, not determined; NR, not reported; SS, high oleic acid sunflower seeds.
* Values in parenthesis indicate the amount of oil in the starter diet fed until 21 d of age before switching to the grower diet fed for 21 d before slaughter.
† Birds received 23 g/lifetime of dried marine microalgae containing 180 g of 22 : 6 n-3/kg.
‡ Control diet contained 50 g/kg of blended vegetable fat that was partially replaced with test lipid supplements.
§ Calculated as the sum of all reported fatty acids.
Similar investigations have been made in chickens (Table 2). Access to pasture or chicory plus a control basal diet was found to alter the FA content of chicken breasts compared with a diet containing linseed and high cis-9 18 : 1 sunflower seed( Reference Azcona, Garcia and Cossu 23 ). Muscle from chickens fed the control plus chicory diet contained higher amounts of n-6 and n-3 PUFA than the control plus pasture diet due to increases in total fat content. Treatments had no effect on FA other than increasing 20 : 5 n-3 abundance. Substituting the control diet for the diet containing linseed and sunflower seed in growing chickens offered access to pasture, decreased total n-6 PUFA and elevated total n-3 PUFA content, including 20 : 5 n-3, 22 : 5 n-3 and 22 : 6 n-3. However, similar changes in muscle FA composition were not observed when birds had access to chicory. Enrichment of 18 : 3 n-3, 20 : 5 n-3, 22 : 5 n-3 and 22 : 6 n-3 in breast meat from chickens fed diets containing flaxseed also increases with time on diet( Reference Mirshekar, Boldaji and Dastar 26 ). Long chain n-3 PUFA content of chicken can also be elevated in birds fed diets containing fish oil and marine algae up to 210 mg/100 g( Reference Kalogeropoulos, Chiou and Gavala 24 , Reference Rymer, Gibbs and Givens 25 ).
Enrichment of PUFA in meat from ruminants
Despite extensive lipolysis and biohydrogenation of lipid in the rumen by the rumen microbiome, diet is the major environmental factor influencing the FA composition of ruminant meat. Forage is an important component in most ruminant diets and can be used to influence the FA composition of beef and lamb. The IMF content and FA composition of beef differs between animals finished on grass or fed concentrates( Reference Ponnampalam, Mann and Sinclair 64 , Reference Aldai, Dugan and Kramer 65 ). Grass-finished beef typically contains higher amounts of 18 : 3 n-3 (Table 3). Depending on management system, 20 : 5 n-3, 22 : 5 n-3 and 22 : 6 n-3 content is also elevated due to the elongation and desaturation of 18 : 3 n-3 in body tissues. Even for animals reared on pasture, feeding concentrates during the finishing period causes depletion of 18 : 3 n-3 and higher accretion of 18 : 2 n-6( Reference Ponnampalam, Mann and Sinclair 64 , Reference Aldai, Dugan and Kramer 65 ). Similar changes in the FA composition of IMF also occur in growing lambs (Table 4). Forages can be used to lower total and saturated fat content and increase the n-3 PUFA content relative to cereal-based diets( Reference Scollan, Dannenberger and Nuernberg 19 , Reference Daley, Abbott and Doyle 68 – Reference Howes, Bekhit and Burritt 70 ).
Table 3. Effect of diet on the fatty acid (FA) composition of beef (mg/100 g)

NR, not reported; SH, Shorthorn; HE, Hereford; AA, Aberdeen Angus; CHX, Charolais cross.
* Control diet contained 31 g/kg diet DM of saturated fat sources that replaced with test lipid supplements.
†Control diet contained a prilled fat rich in 16 : 0 that was partially replaced with a rumen protected source of fish oil.
Table 4. Effect of diet on the fatty acid (FA) content of lamb (mg/100 g)
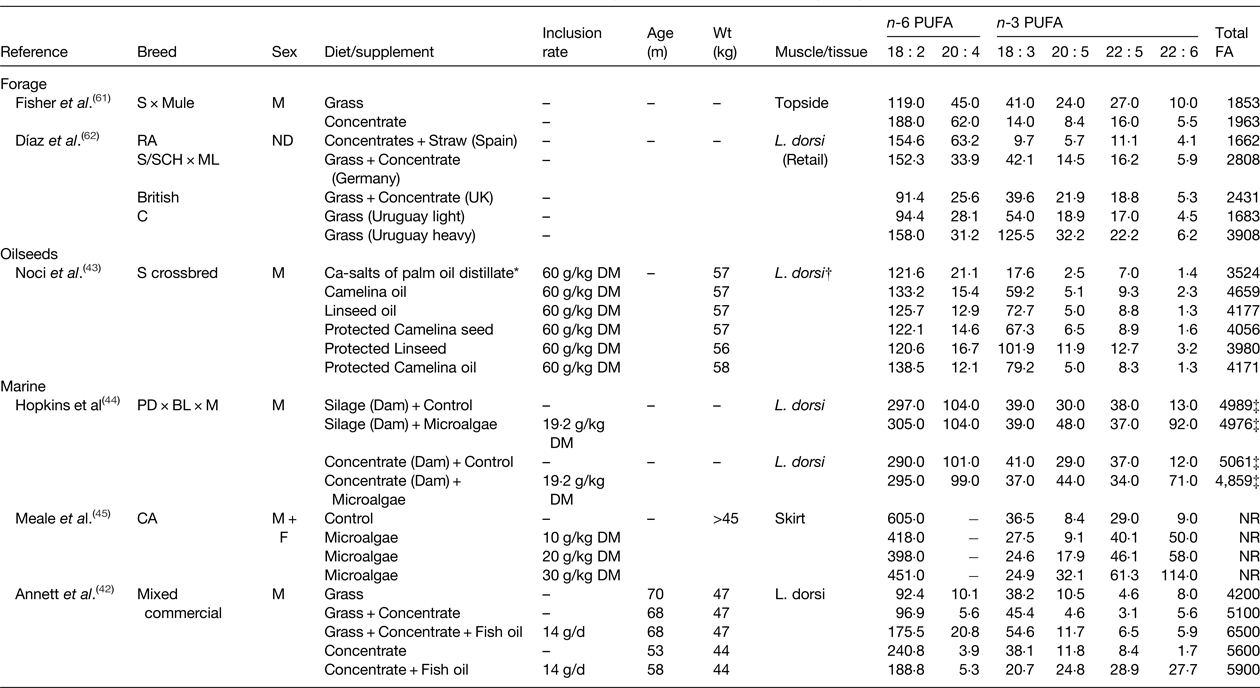
ND, not determined; NR, not reported; S, Suffolk; RA, Rasa Aragonesa; SCH, Schwarzkopfe; ML, Merino Landschaf; C, Corriedales; PD, Poll Dorset; BL, Border Leicester; M, Merino; CA, Canadian Arcott.
* Composition of intramuscular lipid.
† All treatments were fed for 100 d.
‡ Calculated as the sum of all reported fatty acids.
Dietary plant and marine lipid supplements can be used to alter the FA composition of beef (Table 3) and lamb (Table 4). Flaxseed supplementation of a fresh forage basal diet was found to have no effect on n-6 and n-3 PUFA in beef, but the content of trans-11 18 : 1 and cis-9, trans-11 CLA was higher for animals finished on fresh forage compared with a diet containing flaxseed( Reference Pouzo, Fanego and Santini 41 ). Furthermore, flaxseed supplements also increased subcutaneous fat thickness compared with grazing animals. The potential of flaxseed supplementation to alter IMF composition has also been assessed in growing cattle fed a basal diet based on grass-hay or barley-silage( Reference Nassu, Dugan and He 39 ). For both diets, flaxseed lowered 18 : 2 n-6 and 20 : 4 n-6 and increased 18 : 3 n-3 content. Flaxseed had minimal effects on 20 : 5 n-3 and 22 : 5 n-3 content in cattle fed grass hay, but increased 20 : 5 n-3 content and led to marginal enrichment of 22 : 5 n-3 on the barley-silage diet. Further studies have investigated if echium oil, relatively rich in stearidonic acid (18 : 4 n-3), could be used to increase endogenous conversion of C18 n-3 PUFA to long-chain n-3 PUFA( Reference Kim, Richardson and Gibson 38 ), since 18 : 3 n-3 serves as a substrate for Δ6 catalysed desaturation to 18 : 4 n-3 that is considered rate-limiting for the complete desaturation and elongation of 18 : 3 n-3 to 20 : 5 n-3 and 22 : 6 n-3. Supplementing grass-silage with echium oil or linseed oil had no effect on the n-6 or n-3 PUFA content of beef muscle, but increased the abundance of trans-11 18 : 1 and cis-9,trans-11 CLA. Inclusion of camelina oil or linseed oil have proven effective for increasing 18 : 3 n-3 content of IMF in growing sheep, changes that were also accompanied by marginal enrichment of 20 : 5 n-3 and 22 : 5 n-3 compared with a control diet containing calcium salts of palm oil distillate( Reference Noci, Monahan and Moloney 43 ).
The potential of marine sources of PUFA to alter the FA composition of beef has also been investigated. Use of marine algae in combination with either linseed oil or sunflower oil demonstrated that the former elevated 18 : 3 n-3 and the latter increased 18 : 2 n-6 content( Reference Angulo, Hiller and Olivera 40 ). Inclusion of algae with either oilseed increased 22 : 6 n-3 content relative to the control, whereas the abundance of 20 : 5 n-3 and 22 : 5 n-3 were similar among treatments (Table 3). Dietary algae supplements have also been used to enhance long chain n-3 PUFA in lamb. In growing sheep, algae rich in 22 : 6 n-3 resulted in a marginal decrease in 18 : 3 n-3 and dose dependent increases in 20 : 5 n-3, 22 : 5 n-3 and 22 : 6 n-3 content( Reference Meale, Chaves and He 45 ). Changes in maternal nutrition as a mechanism to influence the FA composition of the progeny have been investigated. Nutrition of the dam at mating was found to have minimal effects on lamb FA composition, whereas supplementing the diet of lambs with microalgae increased 22 : 6 n-3, and to a lesser extent, 20 : 5 n-3 content of Latissimus dorsi ( Reference Hopkins, Clayton and Lamb 44 ). The potential of dietary fish oil supplements to alter the FA composition of meat from ruminants has also been examined. Evaluation of different combinations of grass, concentrate and fish oil on lamb FA composition indicated production system (grass or concentrate-based) had a larger influence on overall FA composition than fish oil supplementation, but these findings were based on the feeding of diets with a variable FA content( Reference Annett, Carson and Fearon 42 ). Concentrate-based diets tended to result in the higher deposition of 18 : 2 n-6 in IMF, while fish oil only increased the proportions of 22 : 5 n-3 and 22 : 6 n-3 when included in the high concentrate diet (Table 4).
Most of the research examining the role of nutrition to alter meat FA composition and content have relied on oilseeds and marine lipid supplements that have two major shortcomings, firstly that the amount of supplemental lipid should not exceed 60 g/kg diet DM without affecting performance and secondly that lipid contained in these supplements is metabolised in the rumen. Feeding rumen protected lipid supplements can, to a certain extent, be used to overcome these constraints( Reference Gulati, Garg and Scott 46 ). Non-protected and rumen protected sources of linseed and camelina were shown to alter the FA composition of lamb( Reference Noci, Monahan and Moloney 43 ). In unprotected form, camelina oil and linseed oil increased the 18 : 3 n-3 content of intramuscular fat with evidence of small increases in 20 : 5 n-3 and 22 : 6 n-3. Supplements of protected camelina seeds and linseeds resulted in a higher enrichment of 18 : 3 n-3 compared with unprotected camelina oil and linseed oil, with sodium hydroxide-treated linseeds resulting in the largest increase in 18 : 3 n-3 and long-chain n-3 PUFA content (Table 4). Further studies using a rumen protected fish oil supplement indicated 2-fold increase in 18 : 3 n-3 and a 4-fold increase in 20 : 5 n-3 and 22 : 6 n-3 content in L. thoracis of growing cattle( Reference Dunne, Rogalski and Childs 47 ).
Trans fatty acid content of ruminant meat
High intakes of TFA are associated with increased CVD risk and development of insulin resistance( Reference Mozaffarian, Katan and Ascherio 86 , Reference Brouwer, Wanders and Katan 87 ) and increase inflammation( Reference Mozaffarian 88 ). Concerns over TFA consumption and human non-communicable diseases, has lead to nutritional guidelines( 6 – 8 ), and in some cases legislation( Reference Clarke and Lewington 89 ), recommending a decrease in the TFA content of foods. Such recommendations do not, however, consider differences in the relative abundance and distribution of mono- and polyenoic TFA isomers in ruminant TFA and industrial fats( Reference Shingfield, Chilliard and Toivonen 90 ), other than distinguishing between isomers of CLA containing a trans double bond from other TFA. Enforced or voluntary changes in the refining and processing of plant oils and vegetable fats have decreased the amount of industrial TFA in the human food chain increasing the relative contribution of ruminant TFA to total TFA consumption. Even though there is strong evidence that increases in industrial TFA consumption being associated with mortality from CVD( Reference de Souza, Mente and Maroleanu 91 ), there are insufficient data to conclude on the impact of ruminant TFA intake. Average intake of total TFA in the UK adult population of 0·7 % food energy is below a recommended maximum of 2 % of food energy intake suggesting that present levels of TFA consumption from ruminant foods is not a major CVD risk factor( Reference Lovegrove and Hobbs 92 ).
Ruminant meat contains a range of trans 16 : 1 (∆9–13), trans 18 : 1 (∆4–16) and trans 18 : 2 isomers and trace amounts of 18 : 3 containing one or more trans double bonds ( Reference Kraft, Kramer and Schoene 34 – Reference Bravo-Lamas, Barron and Kramer 37 , Reference Bessa, Alves and Jerónimo 93 – Reference Jerónimo, Alves and Alfaia 95 ). Trans 18 : 1 isomers are quantitatively the most important typically accounting for between 78 and 92 g/100 g total TFA in retail beef and lamb( Reference Kraft, Kramer and Schoene 34 – Reference Bravo-Lamas, Barron and Kramer 37 ). Following absorption, TFA are preferentially deposited in TAG in IMF in contrast to n-3 and n-6 PUFA that are utilised for the synthesis of phospholipids in muscle membranes( Reference Bessa, Alves and Jerónimo 93 , Reference Jerónimo, Alves and Alfaia 95 – Reference Nuernberg, Nuernberg and Ender 97 ). Isomers of TFA in ruminant meat originate from the rumen formed during incomplete conversion of dietary unsaturated FA into saturated end products. In cattle and sheep fed high forage diets trans-11 18 : 1, an intermediate of 18 : 2 n-6 and 18 : 3 n-3 metabolism in the rumen is typically the major TFA leaving the rumen( Reference Shingfield, Wallace, Sels and Philippaerts 98 ). However, high concentrate diets( Reference Piperova, Sampugna and Teter 99 ), starch-rich low fibre rations containing plant oils( Reference Loor, Ueda and Ferlay 100 ) or diets supplemented with high amounts of PUFA( Reference Shingfield, Kairenius and Arölä 84 ) are known to increase the susceptibility to changes in biohydrogenation pathways favouring the synthesis of trans-10 rather than trans-11 intermediates. While a low rumen pH and high dietary concentrations of starch and oil can promote the formation of trans-10 18 : 1 at the expense of trans-11 18 : 1 the underlying causes are not known( Reference Shingfield, Wallace, Sels and Philippaerts 98 ).
Diet has a major influence on the relative abundance of trans 18 : 1 isomers in IMF in beef and lamb (Table 5). In cattle and sheep reared on pasture or fed high forage diets trans-11 is the major 18 : 1 isomer, whereas trans-10 18 : 1 can represent the major TFA in beef or lamb produced on high concentrate diets( Reference Kraft, Kramer and Schoene 34 – Reference Bravo-Lamas, Barron and Kramer 37 , Reference Radunz, Wickersham and Loerch 101 – Reference Alfaia, Alves and Martins 103 ). Even in animals reared on pasture and conserved grass or forage legumes, deposition of trans-10 18 : 1 has been shown to increase during intensive finishing on high concentrate diets (Table 5). Under commercial conditions, the ratio of trans-10 18 : 1:trans-11 18 : 1 in beef or lamb can vary from low values of 0·1 to as much as 20 depending on diet and management system( Reference Bessa, Alves and Santos-Silva 17 ). Studies in several animal models have provided evidence to suggest that trans-10 18 : 1 may have more adverse effects on cardiovascular health compared with trans-11 18 : 1( Reference Salter 104 ). Feeding diets containing plant oils or oilseeds containing cis-9 18 : 1, 18 : 2 n-6 or 18 : 3 n-3 as the major FA can be expected to cause specific enrichment of trans-6–8, trans-10–12, and trans-11–16 in IMF, respectively( Reference Shingfield, Chilliard and Toivonen 90 , Reference Chilliard, Glasser and Ferlay 105 , Reference Mapiye, Turner and Rolland 106 ).
Table 5. Effect of diet on the trans 18 : 1 content of beef and lamb (mg/100 g)
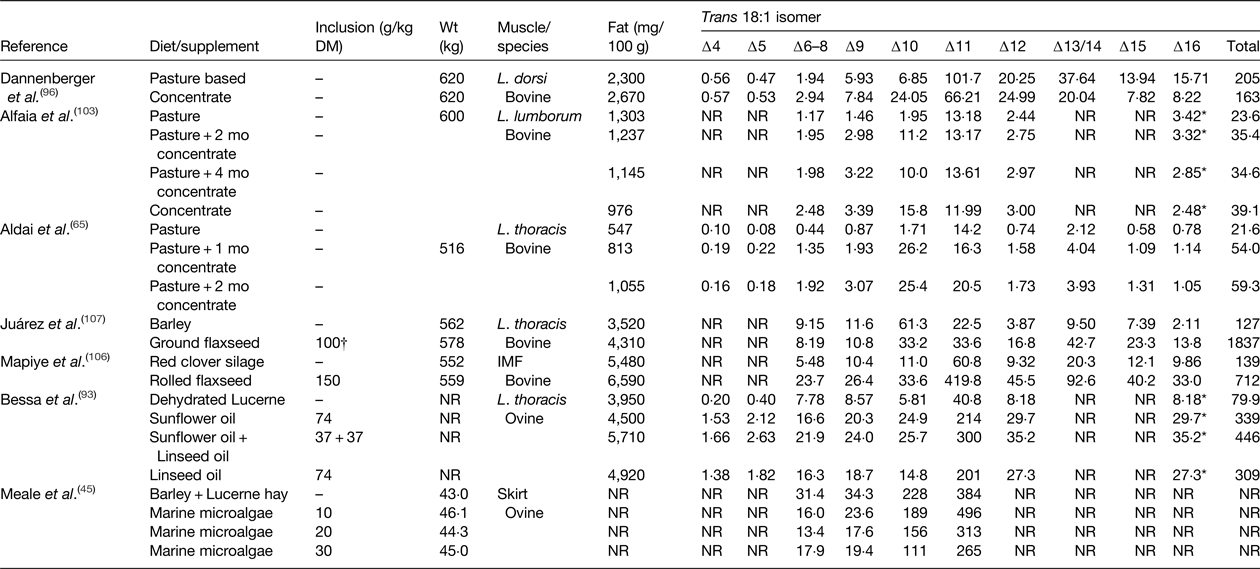
IMF, intramuscular fat; NR, not reported.
* Elutes with the same retention time as cis-14 18 : 1 during gas-chromatography analysis.
† Inclusion rate g/kg as fed.
In cattle or sheep fed forages or cereals, total trans 18 : 2 abundance in IMF varies between 0·51 and 0·70 g/100 g total FA, concentrations that can be increased to 3·0 g/100 g by dietary supplements of oilseeds or plant oils ( Reference Nassu, Dugan and He 39 , Reference Aldai, Dugan and Kramer 65 , Reference Bessa, Alves and Jerónimo 93 , Reference Juárez, Dugan and Aalhus 107 ). Appearance of most trans 18:2 in meat originate from ruminal biohydrogenation of C18 PUFA, but a proportion of cis-9, trans-12 18:2 and cis-9, trans-13 18:2 may also be synthesised endogenously in ruminant tissues( Reference Shingfield, Chilliard and Toivonen 90 ).
Following the identification of the anti-mutagenic properties of CLA isomers in cooked beef( Reference Pariza, Ashoor and Chu 108 – Reference Ha, Grimm and Pariza 110 ), numerous studies have investigated the biological activity of isomers of CLA in cell culture and animal models. Much of the research has focused on the effects of cis-9, trans-11 18 : 2 or trans-10, cis-12 18 : 2. In addition to the inhibition of mutagenesis, specific isomers of CLA have been demonstrated to modulate energy metabolism, immunity, inflammation, insulin resistance and bone metabolism in several animal models, but evidence that the same physiological effects are also replicated in human subjects remains inconclusive( Reference Whigham, Watras and Schoeller 111 – Reference Dilzer and Park 116 ).
Isomers of CLA are present in a wide range of foods including milk, beef and lamb, and in much smaller amounts (0·1 g/100 g lipid) in pork and poultry( Reference Schmid, Collomb and Sieber 117 ). Even though milk and dairy products are the major source in the human diet, lamb, beef and other ruminant meat products contribute to 15–32% of average daily CLA intakes in developed countries( Reference Schmid, Collomb and Sieber 117 – Reference Martins, Lopes and Alfaia 119 ). Ruminant lipid can contain up to sixteen isomers of CLA with double bonds located at 7,9–13,15 depending on diet and production system. Cis-9, trans-11 is typically the major isomer due, in the most part, to endogenous synthesis via the action of stearoyl-CoA desaturase on trans-11 18:1 that accounts for between 45 and 95% of cis-9, trans-11 18 : 2 deposited in muscle and adipose of cattle and sheep( Reference Shingfield, Wallace, Sels and Philippaerts 98 ). Recent studies have also provided evidence that palmitelaidic acid (trans-9 16 : 1) may also serve as a substrate for endogenous cis-9, trans-11 18 : 2 synthesis in ruminant tissues( Reference Kadegowda, Burns and Miller 120 ). Most, if not all, of the trans-7, cis-11 18 : 2 found in ruminant lipid is synthesised endogenously using trans-7 18 : 1 as a substrate( Reference Shingfield, Wallace, Sels and Philippaerts 98 ). Studies in growing lambs and cattle have shown that dietary lipid supplements can be used to enrich cis-9, trans-11 CLA in muscle up to 2·40 g/100 g FA( Reference Sinclair 15 , Reference Shingfield, Bonnet and Scollan 16 ), while inclusion of 60 g sunflower oil /kg DM in the diet of Wagyu cattle with a inherently high IMF content resulted in muscle containing 134 mg cis-9, trans-11 CLA/100 g muscle( Reference Mir, Mir and Kubert 121 ).
Ruminant meat also contain trace amounts of several conjugated linolenic acids that contain at least one conjugated bond( Reference Shingfield, Bonnet and Scollan 16 ). Muscle of growing lambs was reported to contain negligible amounts of cis-9, trans-11, cis-15 18:3, while supplementing the diet with linseed oil over a 42 d finishing period resulting in concentrations of 329 mg/100 g total FA( Reference Bessa, Alves and Jerónimo 93 ). At finishing muscle in cattle contains between 50–239 and 105 mg/100 g total fatty acid methyl esters (FAME) of cis-9, trans-11, cis-15 18:3 and cis-9, trans-13, cis-15 18:3, respectively, and trace amounts (20 mg/100 g total FAME) of cis-9, trans-11, trans-15 18 : 3( Reference Shingfield, Bonnet and Scollan 16 ) Feeding diets containing ground flaxseed over a 140 d finishing period was shown to increase conjugated 18 : 3 content of muscle in beef cows between 30 and 70 mg/100 g total FAME, with evidence that enrichment of specific conjugated linolenic acid isomers is dependent on the composition of the basal diet( Reference Nassu, Dugan and He 39 ).
Potential to alter meat fatty acid content relative to food labelling claims
Numerous studies have explored the potential to alter meat FA composition, with specific emphasis on elevating n-3 PUFA content. It is worth noting altering FA profile has little impact on other aspects of nutrient profile such as protein, vitamins and minerals. The magnitude of increases in n-3 PUFA content that can be achieved can be benchmarked against labelling standards established by the European Food Safety Authority. The established standard are based on reference nutrient intakes and recommended daily intakes for adults of 250 mg 20 : 5 n-3 plus 22 : 6 n-3/d and 2 g 18 : 3 n-3/d( 122 ). Foods should supply >15% of the reference nutrient intakes per 100 g and 418·4 kJ (100 kcal) to be labelled as a ‘source of’, and >30% of the reference nutrient intakes to be labelled as ‘high in’. Meat or meat products must contain ≥40 mg/100 g and per 418·4 kJ (100 kcal) of 20 : 5 n-3 plus 22 : 6 n-3 or ≥0·3 g/100 g and per 418·4 kJ (100 kcal) 18 : 3 n-3 to be labelled as a ‘source of’ n-3 PUFA; or ≥80 mg 20 : 5 n-3 plus 22 : 6 n-3 per 100 g and 418·4 kJ (100 kcal) or ≥0·6 g per 100 g and per 418·4 kJ (100 kcal) 18 : 3 n-3 to be labelled as ‘high in’ n-3 PUFA( 123 ). When interpreting data reported in the literature, n-3 PUFA enrichment is typically reported on a mg/100 g basis, and often the energy content of meat or meat products has not been determined.
Based on amounts of FA (mg/100 g) in muscle for pigs (Table 1) and chickens (Table 2) reared on diets containing linseed or flaxseed it is possible to enrich 18 : 3 n-3 above 0·3 g/100 g. For pigs fed high amounts of flaxseed it is possible to increase 18 : 3 n-3 in pork to 615 mg/100 g( Reference Turner, Mapiye and Aalhus 32 ), a concentration that exceeds the threshold for a ‘high in’ n-3 PUFA claim. At the same time, the increases in muscle total fat content and associated enrichment of 20 : 5 n-3 plus 22 : 6 n-3 also results in pork meeting the requirements for a ‘source of ’ long-chain n-3 PUFA( Reference Turner, Mapiye and Aalhus 32 ). Feeding dietary supplements of fish oil to pigs has also been shown to increase the 20 : 5 n-3 and 22 : 6 n-3 content of pork to levels required to meet the ‘source of’ claim( Reference Haak, De Smet and Fremaut 28 ). Use of fish oil or marine algae can also be used to increase the combined amount of 20 : 5 n-3 and 22 : 6 n-3 in chicken to levels above 80 mg/100 g( Reference Cortinas, Villaverde and Galobart 22 , Reference Kalogeropoulos, Chiou and Gavala 24 , Reference Rymer, Gibbs and Givens 25 ). It is also possible to enrich 20 : 5 n-3 plus 22 : 6 n-3 in muscle to meet the ‘high in long chain n-3 PUFA’ by feeding broilers diets containing flaxseed oil for at least 21 d( Reference Mirshekar, Boldaji and Dastar 26 )and exploiting endogenous conversion of 18 : 3 n-3 to 20 : 5 n-3 and 22 : 6 n-3 in avian muscle.
All the studies outlined in Tables 3 and 4 relating to beef and lamb fail to meet the required ≥0·3 g of α-linolenic acid to even be classed as a ‘source of’ n-3 PUFA. Moreover, the highest 18 : 3 n-3 content for beef was 71·7 mg/100 g in the study by Nassu et al.( Reference Nassu, Dugan and He 39 ) when feeding grass hay and flaxseed. For lamb, the highest 18 : 3 n-3 content was 125·5 mg/100 g with Uruguayan grass-finished heavy lambs( Reference Díaz, Álvarez and De la Fuente 62 ). Nevertheless, some studies did achieve adequate levels of EPA plus DHA to meet ‘a source of’ and ‘high in’ claims. Feeding 275 g/d of protected fish oil resulted in 67·7 mg/100 g of EPA plus DHA in beef, satisfying ‘a source of’ n-3 PUFA' claim( 122 ). Equally, Annett et al ( Reference Annett, Carson and Fearon 42 ) achieved 52·5 mg/100 g EPA plus DHA in lamb fed a fish oil enriched concentrate. Microalgae supplementation of lamb has also been successful in sufficiently increasing EPA plus DHA levels to allow n-3 PUFA health claims. Supplementing diets with 1 and 2% DM microalgae achieved ‘a source of’ levels of EPA plus DHA (59·1 mg/100 g and 75·9 mg/100 g, respectively), while 3% DM supplementation achieved 146·1 mg/100 g EPA plus DHA, which is sufficient to claim ‘high in’ n-3 PUFA( Reference Meale, Chaves and He 45 ). Both control treatments in the study by Hopkins et al ( Reference Hopkins, Clayton and Lamb 44 ) attained ‘a source of’ levels of EPA plus DHA while supplementing these diets with microalgae increase EPA plus DHA levels to above ‘high in’ levels.
While it is helpful to be able to compare relative to labelling standards of European Food Safety Authority, it would be much more useful to assess the relative nutritional value through human intervention studies. There is a distinct lack of this approach in the literature.
Conclusions
Meat provides a range of macro and micronutrients for man. The nutritional value of meat is an important factor influencing consumer preferences for various white and red meats. Substantial progress has been made on reducing the fat content of meat and much effort has focused on approaches for improving FA profile with much emphasis on n-3. Pork and chicken may be enriched with long-chain n-3 by inclusion of fish oil or microalgae in the diet. Enrichment of beef and lamb is more challenging due to the extensive lipolysis and biohydrogenation of dietary lipids by the rumen microbiome. However, some studies have achieved high levels of long-chain n-3 in lamb, sufficient to be noted as high-in n-3 FA according to the guidelines by the European Food Safety Authority. Despite all the efforts to improve lipid profile of meat there is a distinct lack of studies examining the impact through human intervention studies. This essentially helps to make better judgments on the impact of nutritional value on human health and well-being.
Acknowledgements
None.
Financial Support
N. D. S., S. A. H. and K. J. S. have received investment from Biotechnology and Biological Sciences Research Council, Department for Environment Food and Rural Affairs, Agricultural and Horticultural Development Board, Meat Promotion Wales, Quality Meat Scotland and Livestock and Meat Commission and the European Commission.
Conflicts of Interest
None.
Authorship
All authors contributed to the review of published reports. E. M. P., S. A. M. and K. J. S. performed searches of published literature and prepared the Tables summarising information on meat fat composition. All authors reviewed the manuscript contents. N. D. S., S. A. H. and K. J. S. were responsible for the final manuscript contents.








