Introduction
Geographical mapping and analysis of health and healthcare are well-established facets of epidemiological and health services analyses (Joseph and Phillips, Reference Joseph and Phillips1984; Meade and Earickson, Reference Meade and Earickson2005: 381–400), with many studies specifically examining primary health care (PHC; eg, Guagliardo, Reference Guagliardo2004; Luo, Reference Luo2004; Wang and Luo, Reference Wang and Luo2005; Ricketts and Holmes, Reference Ricketts and Holmes2007; Busato and Kunzi, Reference Busato and Kunzi2008). Much of the mainstreaming of health geography has been due to advances in geographic information systems (GIS) methods and technologies, and an associated range of geographically referenced (georeferenced) health and sociodemographic datasets (Samarasundera et al., Reference Samarasundera, Martin, Saxena and Majeed2010a). These trends have also resulted in the growth of interactive, internet-based health and healthcare atlases, as well as software tailored to health services applications, making mapping available to a wider healthcare audience. Examples include the World Health Organization (WHO) mapping and GIS programme (http://www.who.int/health_mapping/en/), the North West England public health observatory mapping facility (http://www.nwpho.org.uk/healthgis/) in the United Kingdom and the New Zealand Ministry of Health intelligence tool (http://www.phionline.moh.govt.nz/).
The increasing application of GIS in PHC is part of this trend. However, making effective use of georeferenced datasets (Samarasundera et al., Reference Samarasundera, Martin, Saxena and Majeed2010a) in GIS software and e-health atlases requires an understanding of geographic concepts (Crooks and Andrews, Reference Crooks and Andrews2009) as well as a managerial understanding of the purposes, scope and limitations of mapping and analysis methods. This review examines spatial mapping and analysis methods and tools for population-level management of PHC and health intelligence purposes, and also discusses the key institutional and implementation issues relevant to effective deployment of such methods and technologies.
Mapping health and health care
Maps are an effective form of visualising information on many topics pivotal to PHC research and development including identifying healthcare access disparities and visualising disease patterns (Walter, Reference Walter1992; Lawson and Williams, Reference Lawson and Williams2001; Cossman et al., Reference Cossman, Cossman, Jackson and Cosby2003). Nevertheless, maps require accurate interpretation and hence understanding both how to design maps effectively and interpret cartographic data are fundamental (Mackie and Sim, Reference Mackie and Sim2008). For example readers might ‘see’ morbidity patterns that do not actually exist due to the effects of the choice of colour scheme and data classes employed (Lawson, Reference Lawson2001).
Colour schemes
A fundamental aspect of visualising cartographic information is the use of colour schemes. Greyscale maps are very popular in academic journals, largely due to cost factors, but are effective in communicating quantitative information, with near-white colours typically representing low levels, and dark grey or black signifying high levels, of a given phenomenon such as disease prevalence. It should be noted that it is considered best practice among many cartographers not to use white to represent low levels, with white itself being reserved to indicate areas for which there are no data. Non-greyscale colour maps of course are also valuable, with single colour gradations and diverging schemes being two standard alternatives for representing numerical gradients. The former is essentially the same as greyscale but uses another colour instead, for example very light pink to very dark red.
Divergent schemes are also common but require more consideration. While map users frequently find the use of a two-colour divergent scheme useful for highlighting very high levels of a phenomenon, it has been found in many cartographic studies that some colour schemes aid visual interpretation while others typically cause confusion. For example there is support for the contention that red–blue colour schemes are generally preferable for disease map construction (eg, Walter and Birnie, Reference Walter and Birnie1991; Brewer and Pickle, Reference Brewer and Pickle2002), other studies having found different schema to be effective, purple–green for example (Brewer et al., Reference Brewer, MacEachren, Pickle and Herrmann1997). A divergent colour scheme that should be discouraged is red–green due to the interpretation difficulties it poses to those with partial colour loss.
Data classification
How data are classified into classes in map legends has long been a key aspect of cartographic design; this is because the choice of class interval types can alter a map user's perceptions of geographic information. There are many alternative classification systems available; common ones are equal interval categories, natural breaks in data, quantiles (eg, quartiles, quintiles, deciles) and classes based on standard deviations. The map producer is thus confronted with what may be a trivial issue but one that can alter the user's interpretation if an inappropriate classification is used. Each of these systems has its uses, given appropriate circumstances. For example equal intervals are useful for making comparisons between different maps of related data. However, as Cromley and Cromley (Reference Cromley and Cromley2009) note, equal interval classifications provide no indication of the statistical distribution of the underlying data; for this, classes based on standard deviations are suitable. Nevertheless, other classification schemes are also useful depending on what point is trying to be communicated in a map (see Indrayan and Kumar, Reference Indrayan and Kumar1996; Brewer, Reference Brewer2006).
A recent cartographic study of public health practitioners using interactive, e-health atlases (Koenig, Reference Koenig2008) has shown that novice health map users may need additional, textual support in interpreting classification systems. PHC informaticians need to consider the expertise level of map users when classifying, visualising and conveying geographic information: providing documentary support for users to aid cartographic communication is imperative. In our view, where there is doubt in classifying data, we recommend the use of quantiles, particularly quintiles, because of their common use in public health for displaying graphical information both in articles (eg, Christie et al., Reference Christie, Morgan, Heaven, Sandifer and van Woerden2005; Adams and White, Reference Adams and White2006) and in internet-based public health atlases.
Map overlay
GIS is essentially the integration of cartography, database technology and geographical analysis. One of its most fundamental facets is map overlay, which refers to the ability to superimpose maps (spatial database layers) of different phenomena upon each other in a GIS package to assess geographic co-location where relevant. A very basic example of map overlay would be superimposing general practitioner (GP) locations (a point layer) onto maps of socio-economic deprivation (area/polygon layers) to visualise the catchment context of a practice. A real-world case in point is the use of map overlay by Nottingham Primary Care Trust (PCT) to compare the geography of provision in New Leaf smoking cessation services (the first layer in the overlay) with that of high-priority target groups defined using geodemographics (the second layer in the overlay), enabling the identification of two highly localised patches of low provision but high needs (Packham and Robinson, Reference Packham and Robinson2009). For further reading on the application of geodemographics in PHC, readers are referred to Powell et al. (Reference Powell, Tapp, Orme and Farr2007), which appeared in Primary Health Care Research & Development. Using a map overlay for this purpose aided a resource allocation rethink such that within a year both patches had improved access to cessation services.
Many examples of the use of overlay techniques in epidemiological and health services analyses exist (eg, Luo, Reference Luo2004; Wang and Luo, Reference Wang and Luo2005). For instance, Field (Reference Field2000) used a map overlay of indicators of population morbidity, socio-economic status, transport access and built environment quality to produce an Index of Relative Disadvantage to identify localities with higher healthcare needs co-located with lower accessibility to GP clinics. Overlay operations typically involve bringing two layers together to form a new dataset made of some combination of the first two layers, for example to identify how many clinics (an example of a point layer) are within a census tract (termed a polygon layer in GIS terminology) so as to create a new dataset consisting only of the shared areas between the two original layers. Another common technique is the union operation, which enables the creation of a new layer consisting of all areas in the original two layers combined, such as when adjoining neighbouring census tracts to produce a larger area. Table 1 summarises some common overlay operations, their purposes and example PHC applications.
Table 1 Some common overlay operations, their purposes and example primary health care applications
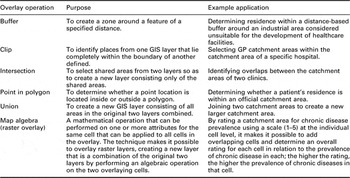
GIS = geographic information systems; GP = general practitioner.
Spatial analyses
Beyond basic mapping, the use of GIS in PHC also extends to analytic purposes, which can vary from assessing access to services, to identifying patterns in clinical outcomes, to producing local estimates of population disease risk. In this section, relevant methods are critiqued in two sub-sections pertaining to two core concepts from the health services domain of the Faculty of Public Health, three domains of the public health paradigm (Griffiths et al., Reference Griffiths, Jewell and Donnelly2005), clinical effectiveness and services planning, respectively, as foundations for efficiency, audit and evaluation ultimately aiming towards equity.
Analytic methods for investigating clinical effectiveness
Detecting clusters and outliers are novel and growing aspects of spatial analysis in PHC. In relation to routine surveillance and regulation, it is in essence the ability to detect statistically significant spatial clusters and outliers in service performance, as well as disease (risk) hotspots. There are a range of different techniques to this end including Local Moran's I, Getis Ord Gi* statistic and Geary's C (Cromley and McLafferty, Reference Cromley and McLafferty2002: 130–57). The key concept to understand is that all of these measures are based on the comparison of the value of a given variable (eg, prevalence of coronary heart disease, CHD) at a specified location with values at proximate locations up to a specified threshold distance, in what is technically termed spatial autocorrelation. Thus, statistically significant clusters (hotspots) and outliers (coldspots) can be detected, and this ability should be distinguished from clusters suggested by eye gazing a map; subjective visual perceptions can sometimes suggest false clusters and outliers as commented on in the opening to the section on mapping health and health care. A primary care application of spatial cluster/outlier detection can be found in Nacul et al. (Reference Nacul, Soljak, Samarasundera, Hopkinson, Lacerda, Indulkar, Flowers, Walford and Majeed2011) in which the Local Moran's I test was used to compare observed PHC-based prevalence of chronic obstructive pulmonary disease with estimated prevalence based on epidemiological models for the purpose of identifying clusters of under-diagnosis for resource allocation purposes.
A caveat to the use of cluster/outlier detection techniques, however, is the existence of the modifiable areal unit problem, a prime but often neglected issue that refers to the effect that observed patterns can potentially change at different geographic scales (the scalar effect, a form of data aggregation/disaggregation effect) and with the use of different areal units (the zonal effect). However, it should be noted that Stafford et al. (Reference Stafford, Duke-Williams and Shelton2008) used self-reported health datasets on 1550 people at the individual postcode level, from the 1999 Health Survey for England, aggregated to three alternative small area configurations – firstly census wards, secondly delineations according to built environment and thirdly units based on housing type – and found only a minor variation in the patterns observed between the three configurations. This suggests that the zonal effect might be negligible with respect to the type of routine health data used in PHC analyses. Nevertheless, the choice of spatial scale appears to be important as shown by comparison between the findings of Samarasundera et al. (Reference Samarasundera, Soljak, Indulkar and Calderon2010b) and Soljak et al. (Reference Soljak, Samarasundera, Indulkar, Walford and Majeed2011) regarding clustering of stroke under-diagnosis in primary care reveals. The Soljak et al. study examined patterns at the PCT level, finding evidence for significant clustering of under-diagnosis across Greater London using PCT-level data; however, parallel work by Samarasundera et al. (Reference Samarasundera, Soljak, Indulkar and Calderon2010b) using practice-level datasets largely yielded significant clusters for practices in inner London, with few clusters remaining for outer London at the practice level. The lesson to learn from this and other studies on the same topic (eg, Schuurman et al., Reference Schuurman, Bell, Dunn and Oliver2007; Callaghan et al., Reference Callaghan, Cormican, Prendergast, Pelly, Cloughley, Hanahoe and O'Donovan2009) is that it is important to perform cluster/outlier detection at more than one scale to assess scalar effects.
However, cluster/outlier detection is not the only spatial analytic approach relevant to assessing clinical effectiveness. Spatial forms of regression, termed spatial regression, also have relevance. Traditional regression has a long history in health-related analyses, including in health geography, to evaluate health outcomes in relation to other variables such as socio-economic status. An illustrative example of the application of traditional regression analysis in a PHC context is Busato and Kunzi (Reference Busato and Kunzi2008). In the study, consultation rates were regressed against health care supply and sociodemographic factors in multivariate models for localities across Switzerland, enabling the researchers to assess the impacts of local contextual factors for utilisation behaviour.
While traditional regression is well suited for many analytical purposes, it is nevertheless non-spatial in structure and hence does not account for the effects of spatial proximity (eg, between adjacent census tracts) or boundary effects (eg, census tract boundaries are convenient but artificial, and thus rarely represent actual physical boundaries) as has been considered in the previous sub-section. As a result, those engaged in more complex analyses are increasingly using spatially explicit regression methods. While each method of spatial regression differs, there is a core similarity in that they all divide datasets into geographic sub-samples and perform local regression around each regression point (ie, data location) rather than on the full dataset. Sub-samples are defined based on a statistical matrix based on the data in question, a process typically implemented in software packages automatically using spatial algorithms.
A spatial regression approach gaining increasing attention is the use of spatial lag and error models (Anselin, Reference Anselin1988), which although initially developed in the disciplines of spatial econometrics and economic geography, has been found to be useful in many other application areas including the health sector (eg, Emch et al., Reference Emch, Ali, Park, Yunus, Sack and Clemens2006; Mobley et al., Reference Mobley, Root, Anselin, Lozano-Gracia and Koschinsky2006). A primary care application of this method is demonstrated by Mobley et al. (Reference Mobley, Root, Anselin, Lozano-Gracia and Koschinsky2006), who used spatial lag and error regression to identify localised variations in the effects of PHC factors and area context on hospital admissions for primary care sensitive conditions (PCSC) such as asthma and ear infections. Higher local rates in PCSC than the national average may indicate, to continue with the same disease examples, unmanaged asthma and inadequately treated ear infections. Indeed, one of the key findings of the study was that localities with lower levels of clinical support for GPs (eg, practice nurses) correlated strongly with higher admission rates. It is worth stressing that this correlation became more apparent in the Mobley et al. (Reference Mobley, Root, Anselin, Lozano-Gracia and Koschinsky2006) study with the use of spatial lag and error models than with classical regression models, and greatly aided the locality triangulation process. A point to consider nonetheless is that there is not always a spatial structure in regression relationships (technically termed spatial autocorrelation) and, under these circumstances, traditional regression will work just as effectively. While it may seem a daunting task to know when to use traditional regression and when to use spatial regression, the relevant software packages (see the sub-section on GIS and spatial statistics software for relevant websites) always include at least one test for spatial autocorrelation as a form of good practice.
Analytic methods for services planning
Perhaps the most obvious geographical aspect of PHC analysis is assessing spatial accessibility, which effectively is essential for service planning. Network analysis plays a fundamental role in this respect (eg, Wang and Luo, Reference Wang and Luo2005) as it allows the travel distance from a particular postcode or the centre point of a census tract (centroid) to the nearest GP to be calculated using road, or other transport, networks and offers a level of sophistication beyond the more simplistic approach of using straight line (Euclidean) distances that many studies have used successfully (eg, Damiani et al., Reference Damiani, Propper and Dixon2005; Propper et al., Reference Propper, Damiani, Leckie and Dixon2007). It is important to note that Euclidean distance does not always reflect the actual distances travelled to access health care using network methods and analysts should compare both measures whenever feasible as Jordan et al. (Reference Jordan, Roderick, Martin and Barnett2004) have demonstrated.
Typical GIS software also allows the calculation of drive times based on speed limits for given stretches of roads. Furthermore, it is possible to select specific roads required in the analysis depending on the criteria, for example the Jordan et al. (Reference Jordan, Roderick, Martin and Barnett2004) study incorporated primary, secondary and minor roads, given that these would typically be utilised when accessing a local GP clinic with roads such as motorways excluded. Their research concluded that drive time is a more accurate measure of access to health care especially in rural areas when compared with Euclidean distance. Network analysis can also be combined with spatial regression techniques for investigating accessibility and utilisation patterns, to explore how local variations in travel time vary in relation to localised pockets of deprivation in order to identify ‘hotspots’ of co-variation in poor spatial accessibility with less economically advantaged communities. Indeed, a growing number of studies have shown that doing so provides greater insights than traditional regression methods (Dawe, Reference Dawe2008; Bagheri et al., Reference Bagheri, Holt and Benwell2009; Jattansingh, Reference Jattansingh2009; Walsh, Reference Walsh2009).
A further analytic method relevant to service planning is kernel density smoothing, which is used to interpolate between point-based data (eg, practice locations) or to smooth census tract estimates of a phenomenon (eg, attendance rates) to account for boundary effects (smoothing is the re-estimation of the observed value at a tract based on not only the observed value but also its neighbours). Sociodemographic boundaries are by and large convenient abstractions rather than representing real-world boundaries. However, their use in analytical research can sometimes introduce problems. While for most routine monitoring purposes artificial boundaries are not a significant issue, the issue can sometimes become problematic when attempting to model processes and patterns, for example in healthcare utilisation behaviour. Smoothing techniques also have relevance to interpolating values between locations to provide estimates for places where data have not been collected or are unavailable.
One application of such a technique by Guagliardo (Reference Guagliardo2004) used kernel density smoothing in order to investigate the spatial accessibility of primary care. Here, a continuous density layer was created from points representing physician accessibility across a city. The kernel density method made it possible to calculate the average physician density for each neighbourhood, with smoothing allowing for census tract border crossing to be accounted for. A similar method was adopted by McLafferty and Grady (Reference McLafferty and Grady2004; Reference McLafferty and Grady2005), whereby prenatal care and access are analysed using kernel density estimation to establish the density of prenatal clinics in relation to mothers’ residential locations. Figure 1 illustrates the use of kernel density smoothing to produce a continuous disease map using practice-level point pattern data in the form of Quality Outcomes Framework-based (Sigfrid et al., Reference Sigfrid, Turner, Crook and Ray2006; Department of Health, 2008: 9) decile-based prevalence estimates for CHD in northern England.
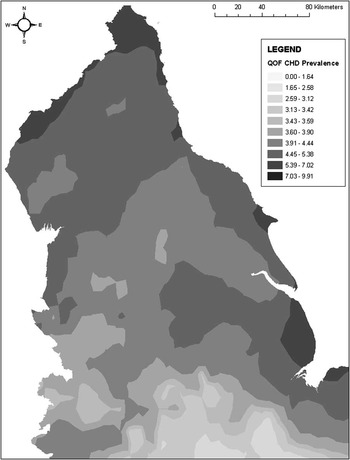
Figure 1 Predicted coronary heart disease prevalence based on an inverse-squared distance kernel density model
The limitation of kernel density approaches is that the shape of the kernel is inherently arbitrary and does not consider the spatial arrangement of the data in question, such that localities with a high density of points are smoothed in the same way as those with sparse points. Hence, more sophisticated interpolation methods such as geostatistics (commonly termed kriging) are sometimes appropriate, although the use of such techniques is beyond the scope of routine PHC mapping and analysis and are hence not critiqued here. For an introduction to geostatistics in health, readers are referred to Cromley and McLafferty (Reference Cromley and McLafferty2002: 200–204); advanced readers are referred to Webster and Oliver (Reference Webster and Oliver2007).
Software, the internet and organisational issues
There are a range of packages available for mapping and analyses to implement the methods discussed thus far in this review, with different software types having different purposes and different specific packages having differing strengths and weaknesses. In addition to these desktop programs, there are also a range of internet-based sites offering mapping and sometimes analytic functionality. Internet-based GIS facilities offer no- or low-cost solutions requiring little or no domain expertise for basic health surveillance and services planning; more complex analytic work of the type previously discussed with respect to tasks such as identifying spatial variation in under-diagnosis and interpolating clinic-level prevalence datasets requires domain knowledge and specialist software. Selecting the fit-for-purpose tools and the associated institutional considerations are discussed in this section.
Internet-based mapping
With respect to core mapping of local indicators, the growth of interactive, e-health atlases underpinned by GIS software has provided health intelligence with valuable routine locality profiling tools. Many of these atlases and tools are operated by public health bodies, for example the New Zealand Ministry of Health Public Health Intelligence (http://www.phionline.moh.govt.nz/) and the North West Public Health Observatory Health GIS (http://www.nwpho.org.uk/healthgis/). Such atlases are typically produced using cut-down GIS packages such as InstantAtlas (http://www.instantatlas.com/), which require little domain expertise other than familiarity with spreadsheets, and are thus readily deployable in PHC settings without significant additional staff training, outsourcing or buying in of new expertise. The functionality provided by such packages invariably enables users to link to graphical data such as line graphs on time trends in disease for a given area or funnel plots for locating performance outliers.
Some of these public health atlases include datasets specific to PHC, a case in point being the New Zealand Ministry of Health Public Health Intelligence interactive atlas (http://www.phionline.moh.govt.nz/), which includes a primary care section summarising data on PHC-based prevalence, utilisation and unmet needs. The facility allows users, for example, to identify unmet needs in a primary care organisation by clicking on its name in the accompanying table. Doing so then highlights that organisation on the map and on the accompanying graph so that comparisons can be made with neighbouring bodies.
Value-added tools and customised services
Similar mapping functionality is available for many internet- and network-based tools and services such as the National Health Service (NHS) Strategic Health Asset Planning and Evaluation (SHAPE) mapping facility in the United Kingdom (http://shape.dh.gov.uk/public/mapping.asp), HealthLandscape Primary Care Atlas in the United States of America (http://www.healthlandscape.org/) and iQ HealthMaps in the United Kingdom (http://www.iq-medical.co.uk/products-healthmaps.php). Such tools are typically customised, internet-enabled GIS software that offer not only basic mapping facilities but also some spatial analytic functionality and often include useful datasets within them; hence, they can be considered as value-added tools. For example iQ HealthMaps integrates Google-based mapping with the ability to perform basic point pattern mapping of patient residences and also allows the overlay of integrated datasets such as MOSAIC geodemographic classifications (see Webber, Reference Webber2004 for a discussion on the use of MOSAIC in health services analysis), aspects of the built environment and healthcare infrastructure without the need for specialist skills on the part of the user. Both SHAPE and iQ HealthMaps offer users the ability to explore scenarios such as broad-level impacts of the closure and opening of new healthcare facilities upon communities by overlay different GIS layers such as morbidity rates, drive-time distances and sociodemographic data. While broadly similar in facilities, nevertheless, some clear distinctions can exist even between value-added tools. For example among the examples already cited, SHAPE is state-run and free to NHS staff whereas iQ HealthMaps is a commercial product; HealthLandscape Primary Care Atlas and its associated tools are free to a certain extent but are at a cost for their more sophisticated facilities.
The examples discussed above are but some of a range of tailored solutions available, Managers of PHC information should carefully consider whether it is cartographic visualisation (eg, mapping disease trends) or spatial analysis (eg, calculating drive times for accessibility analysis) that is more relevant from their organisational perspective, and exactly what functionality they require in order to successfully co-ordinate the use of different GIS solutions products as part of fit-for-purpose healthcare intelligence systems.
GIS and spatial statistics software
For those involved (or wishing to develop capacity) in spatial analytic work such as statistically based cluster/outlier detection, complex desktop-based packages are a fundamental addition to the range of tools already discussed. As GIS continues to penetrate healthcare research and development, it is likely that spatial analysis will be the next geography-related trend in primary care informatics to complement and build on the current proliferation of healthcare mapping, although currently full analytical packages are largely used by epidemiologists and many organisations in the public health sector (eg, health protection bodies) rather than by those focusing on PHC. This is especially likely in countries such as the United Kingdom, where Joint Needs Assessment collaborations with local governmental authorities linked to urban renewal (Blackman, Reference Blackman2006; National Health Service London Healthy Urban Development Unit, 2006; Land Use Consultants, 2007) require integrated, medium-term spatial planning.
There is a range of relevant software for analytical purposes including mainstream GIS software such as ESRI ArcGIS (http://www.esri.com/software/arcgis/index.html) and MapInfo (http://www.pbinsight.eu/uk/products/location-intelligence/), which also provide mapping and spatial database management facilities, as well as more specialist spatial statistics packages such as GeoDa (http://geodacenter.asu.edu/geodasum) and GWR (http://ncg.nuim.ie/ncg/GWR/software.htm). PHC informaticians, managers and planners working at highly local community levels are unlikely to need such software, at least in the context of the majority of More Economically Developed Countries (MEDCs), considering the range of tailored software and internet mapping presently available in such parts of the world. However, PHC researchers and developers working with larger datasets and with greater analytic needs, such as those planning at the strategic level, should seriously consider deploying such tools if this has not been done already. In the second main section of this review, the use of cluster/detection techniques, spatial regression and interpolation was discussed in relation to primary care, but what about deciding whether to actually deploy the relevant software within a given organisation? A not uncommon mistake, across many employment sectors, is to introduce complex geographical software without (a) an overall strategy and goals in mind and (b) without appropriately trained personnel. Also, the appropriate use of statistical techniques always requires domain knowledge and should be viewed as a distinct activity in itself. A GIS package is suitable when an organisation requires significant in-house management of spatial data as well as the abilities to perform cartographic and analytic work. Spatial statistics packages can often be a valuable foil to the use of GIS software for such purposes, especially in contexts where visual identification of clusters/outliers is insufficient, thus requiring more formal methods of detection and for whom statistical triangulation of PHC performance indicators is the core activity.
Nevertheless, relatively recent studies of the use of such tools in healthcare organisations in MEDCs reveal much room for further productive uptake of spatial analytic tools. For example Smith et al. (Reference Smith, Gould and Higgs2003) and Higgs et al. (Reference Higgs, Smith and Gould2005) researched GIS use in the British NHS, and while the studies showed increasing awareness of the benefits of such tools, no doubt a contributing factor to the present usage levels of internet-based mapping and value-added tools, a key obstacle was found to be limited data sharing between healthcare bodies. This hurdle was found to be more evident regarding data sharing between the healthcare sector and local authorities, a source for concern considering the importance of collaborative health improvement strategies. With respect to the types of PHC-relevant spatial analysis being carried out in the NHS, the Smith et al. study was revealing in that much of the analytic uses of GIS in 2001 are little different from those we can observe today, the most common applications being for monitoring locality healthcare needs and planning catchment areas. There was little evidence of the use of GIS for modelling purposes and forecasting, a shortfall that is gradually changing, although it is the private sector that has successfully come to dominate this growth niche and aid in taking this invaluable agenda forward in the United Kingdom (eg, Dr Foster Population Health Manager: http://www.drfosterintelligence.co.uk/managementInformation/PHM/).
An interesting strategic solution to the organisational implementation of spatial analysis is that adopted in New Zealand, where the Ministry of Health has developed a joint initiative with the University of Canterbury to set up the GeoHealth Laboratory, which provides ongoing geographical intelligence for both disease monitoring and care development (http://www.geohealth.canterbury.ac.nz/; Pearce, Reference Pearce2007); some of the laboratory's work directly relates to primary care (eg, Hiscock et al., Reference Hiscock, Pearce, Blakely and Witten2008) although the laboratory's remit extends to the wider health services infrastructure as well as to epidemiological studies.
The situation is somewhat complicated in Lesser Economically Developed Countries, with limited availability of the relevant tools through PHC in the ‘Third World’, which frequently overlaps with health protection issues relating to infectious disease epidemiology anyway, can follow the lead of environmental health specialists by making use of Google Maps and Google Earth for basic mapping (eg, Lozano-Fuentes et al., Reference Lozano-Fuentes, Elizonda-Quiroga, Farfana-Ale, Lorono-Pino, Garcia-Rejon, Gomez-Carro, Lira-Zumbardo, Najera-Vazquez, Fernandez-Salas, Calderon-Martinez, Dominguez-Galera, Mis-Avila, Morris, Coleman, Moore, Beaty and Eisen2008) and combining their use with some of the freeware analytic packages available such as GRASS and GeoDa. Interfacing by the PHC sector in such countries with non-governmental organisations and the WHO with respect to developing GIS capacity could be a potentially invaluable contribution to the meeting of stated WHO goals in programmes such as the African Healthy Cities (World Health Organization, 2003) and Closing the Gap in a Generation (World Health Organization, 2008) programmes.
Summary and future directions
The mainstreaming of spatial mapping and analysis techniques and toolkits has provided the means by which to utilise a growing range of routinely available geographic data sources for PHC research and development. However, careful consideration of resource issues and strategic goals is paramount for successful implementation within an organisation.
Developing collaborative networks within the health sector as well as with academic institutions can greatly aid this agenda, as evidenced by the role of the Association for Geographic Information (AGI) Local Public Services Special Interest Group (http://www.agi.org.uk/lps-welcome). Data sharing and interdisciplinary work would be essential for any such networks to be fruitful, topics previously raised in environmental health circles as being important to progress GIS use further in that area of health (Dunn et al., Reference Dunn, Bhopal, Cockings, Walker, Rowlingson and Diggle2007). The biggest obstacle to this goal appears to be a lack of awareness of the potential of GIS for analysis and modelling, and even the existence of spatial statistics, within health services organisations including at senior levels (Smith et al., Reference Smith, Gould and Higgs2003; Higgs et al., Reference Higgs, Smith and Gould2005; Joyce, Reference Joyce2009), and this may be the first thing that needs to be addressed. Organisations such as the United Kingdom's AGI and its equivalents in other countries will be pivotal to the growth of primary care GIS.
With the topic of developing the geographical mapping and analysis agenda in mind, what aspects of this agenda need further research and development? Certainly GIS is well established within epidemiology, health services and public health at least to the extent that there are standard textbooks on the subject (eg, Cromley and McLafferty, Reference Cromley and McLafferty2002; Craglia and Maheswaran, Reference Craglia and Maheswaran2004; Skinner, Reference Skinner2010) but what of the specific case of primary care? Starting from the literature base, while there are certainly many public health journals that publish geographically related articles, as well as two journals specialising in health geography (Health and Place, International Journal of Health Geographics), a search for the terms ‘primary health care’ or ‘primary care’ in co-occurrence with GIS-related terms reveals a dearth of literature pertaining to statistical, internet-based tools explicitly for PHC development. This literature gap mirrors the tools gap, with the exception of Dr Foster Population Health Manager, which, in many respects, is moving in this direction.
Nevertheless, there are areas in which synergy between PHC bodies, public health intelligence and academia could develop internet-based tools for the routine use of statistical cluster/outlier detection and spatial regression. While actual processing tools for such techniques would be too computationally intensive for current internet technology, a useful step forward would be the development of interactive atlases that provide only the results of spatial analysis pertaining to primary care data. A hypothetical example of this could be an interactive atlas of clusters/outliers in modelled under-diagnosis in PHC across a range of chronic conditions, utilising the results of analytic studies such as Nacul et al. (Reference Nacul, Soljak, Samarasundera, Hopkinson, Lacerda, Indulkar, Flowers, Walford and Majeed2011). Such a tool could aid practices and regulatory bodies in identifying disease priorities for service development and compare performance with neighbouring institutions.
Geographical mapping and analysis aimed specifically at PHC is still at an early stage. To demonstrate its potential for cutting-edge development to those working in the ‘front line’ of informing and managing service delivery, collaboration between academics working, respectively, in GIS and PHC to produce proof-of-concept demonstrator systems for analytic, internet-based PHC tools might be the best starting point.




