I. INTRODUCTION
Butenafine hydrochloride (sold under the brand names Lotrimin Ultra and Mentax) is the hydrochloride salt form of butenafine, a synthetic benzylamine derivative with fungicidal properties. It is used to treat athlete's foot, ringworm, and jock itch. With extended use, it can have some benefit in treating toenail fungus. The systematic name (CAS Registry Number 101827-46-7) is 1-(4-tert-butylphenyl)-N-methyl-N-(naphthalen-1-ylmethyl)methanamine hydrochloride. A two-dimensional (2D) molecular diagram is shown in Figure 1.

Figure 1. The 2D molecular structure of racemic butenafine hydrochloride.
Conformational analyses of both butenafine free base and its protonated cation have been carried out (Mingeot-Leclercq et al., Reference Mingeot-Leclercq, Gallet, Flore, Van Bembeke, Peuvot and Brasseur2001). We are unaware of any published X-ray powder diffraction data on butenafine or butenafine hydrochloride.
This work was carried out as part of a project (Kaduk et al., Reference Kaduk, Crowder, Zhong, Fawcett and Suchomel2014) to determine the crystal structures of large-volume commercial pharmaceuticals, and include high-quality powder diffraction data for them in the Powder Diffraction File (Gates-Rector and Blanton, Reference Gates-Rector and Blanton2019).
II. EXPERIMENTAL
Butenafine hydrochloride was a commercial reagent, purchased from TargetMol (Lot #118887), and was used as-received. The white powder was packed into a 1.5-mm diameter Kapton capillary, and rotated during the measurement at ~50 Hz. The powder pattern was measured at 295 K at beamline 11-BM (Antao et al., Reference Antao, Hassan, Wang, Lee and Toby2008; Lee et al., Reference Lee, Shu, Ramanathan, Preissner, Wang, Beno, Von Dreele, Ribaud, Kurtz, Antao, Jiao and Toby2008; Wang et al., Reference Wang, Toby, Lee, Ribaud, Antao, Kurtz, Ramanathan, Von Dreele and Beno2008) of the Advanced Photon Source at Argonne National Laboratory using a wavelength of 0.458968(2) Å from 0.5 to 50° 2θ with a step size of 0.001° and a counting time of 0.1 s/step. The high-resolution powder diffraction data were collected using twelve silicon crystal analyzers that allow for high angular resolution, high precision, and accurate peak positions. A silicon (NIST SRM 640c) and alumina (SRM 676a) standard (ratio Al2O3:Si = 2:1 by weight) was used to calibrate the instrument and refine the monochromatic wavelength used in the experiment.
The pattern was indexed using both JADE Pro (MDI, 2022) and N-TREOR (Altomare et al., Reference Altomare, Cuocci, Giacovazzo, Moliterni, Rizzi, Corriero and Falcicchio2013) on a primitive monoclinic unit cell with a = 13.92505, b = 9.09245, c = 16.43938 Å, β = 93.97°, V = 2076.45 Å3, and Z = 4. A reduced cell search in the Cambridge Structural Database (Groom et al., Reference Groom, Bruno, Lightfoot and Ward2016) yielded 8 hits, but no structures of butenafine derivatives. The suggested space group was P21, which was confirmed by successful solution and refinement of the structure.
A butenafine molecule was downloaded from PubChem (Kim et al., Reference Kim, Chen, Cheng, Gindulyte, He, He, Li, Shoemaker, Thiessen, Yu, Zaslavsky, Zhang and Bolton2019) as Conformer3D_CID_2484.sdf. It was converted to a *.mol2 file using Mercury (Macrae et al., Reference Macrae, Sovago, Cottrell, Galek, McCabe, Pidcock, Platings, Shields, Stevens, Towler and Wood2020) and into a Fenske-Hall Z-matrix using OpenBabel (O'Boyle et al., Reference O'Boyle, Banck, James, Morley, Vandermeersch and Hutchison2011). The structure was solved by Monte Carlo simulated annealing techniques as implemented in EXPO2014 (Altomare et al., Reference Altomare, Cuocci, Giacovazzo, Moliterni, Rizzi, Corriero and Falcicchio2013) using two butenafine molecules and two Cl atoms as fragments. Hydrogen atoms were added to the two N atoms using Materials Studio (Dassault, 2021).
An initial refinement of 181 variables using 18,073 observations and 126 restraints yielded the residuals R wp = 0.1378 and GOF = 2.49 (Figure 2). Although the root-mean-square (rms) Cartesian displacement between the Rietveld-refined and DFT-optimized structures of cations 1 and 2 (0.106 and 0.196 Å, respectively; Figures 3 and 4) is within the normal range for correct structures (van de Streek and Neumann, Reference van de Streek and Neumann2014), the Rietveld fit is not as good as one might hope and the structure is chemically unsatisfactory. Only one of the cations (cation 1; the lower atom numbers) forms the expected N–H⋯Cl hydrogen bond; the N1⋯Cl104 distance was 3.061 Å (Figure 5). The corresponding N52⋯Cl103 distance for cation 2 was 3.619 Å. The conformational study of Mingeot-Leclercq et al. (Reference Mingeot-Leclercq, Gallet, Flore, Van Bembeke, Peuvot and Brasseur2001) noted that, although the butenafine free base molecule is achiral, protonation can result in both R and S enantiomers. Both molecules were in the R configuration, as they were generated from two copies of the downloaded molecule. Manually inverting the configuration of N52 from R to S using Materials Studio and performing a molecular mechanics-based structure optimization using the Forcite module yielded the initial model for a much more satisfactory Rietveld refinement. Butenafine hydrochloride turns out to crystallize as a racemic co-crystal of enantiomers, explaining the Z′ value of 2.
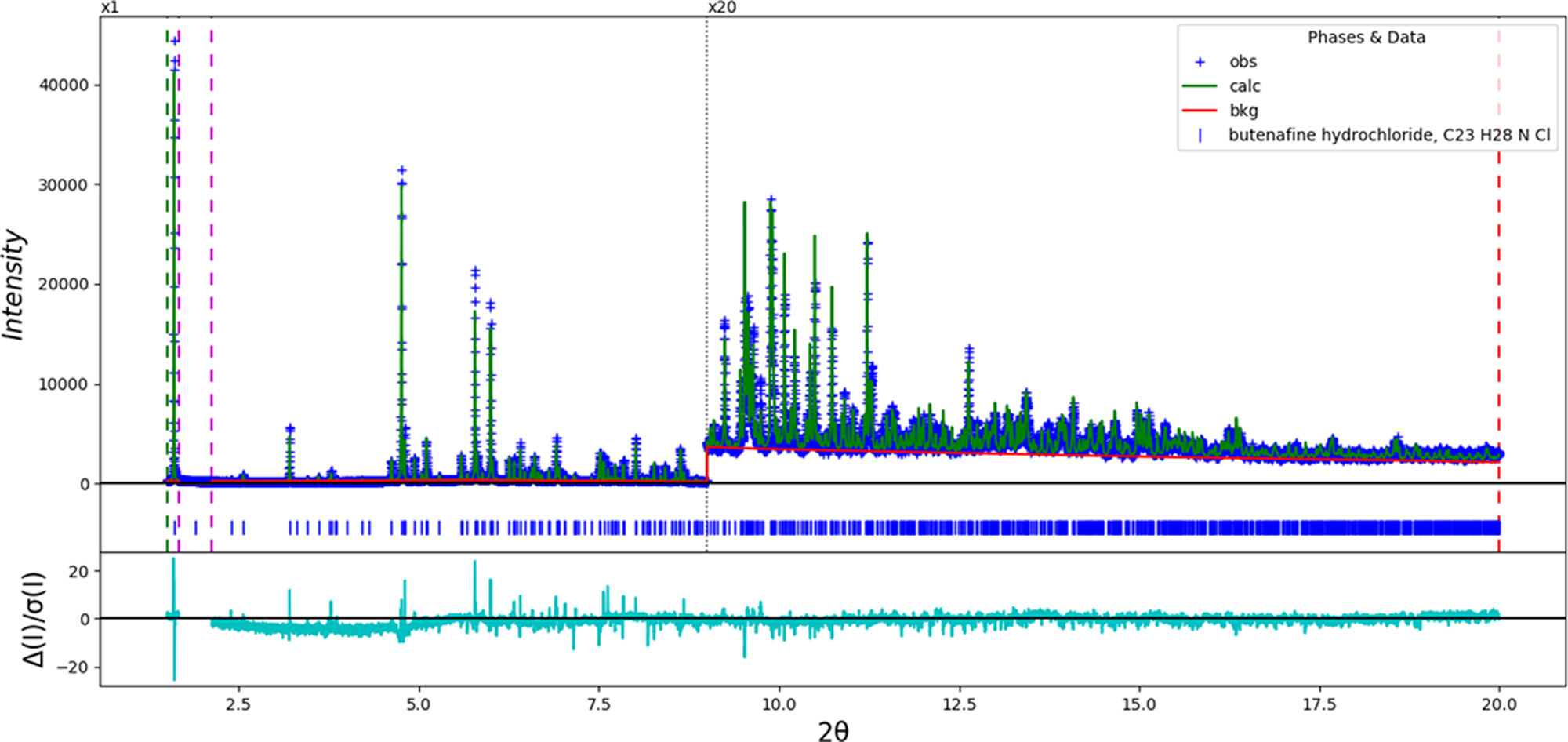
Figure 2. The Rietveld plot for the first (incorrect) refinement of butenafine hydrochloride. The blue crosses represent the observed data points, and the green line is the calculated pattern. The cyan curve is the normalized error plot. The red curve indicates the background. The vertical scale has been multiplied by a factor of 20× for 2θ > 9.0°. The row of blue tick marks indicates the calculated reflection positions.

Figure 3. Comparison of the Rietveld-refined (red) and VASP-optimized (blue) structures of cation 1 in butenafine hydrochloride from the first (incorrect) refinement. The rms Cartesian displacement is 0.106 Å. Image generated using Mercury (Macrae et al., Reference Macrae, Sovago, Cottrell, Galek, McCabe, Pidcock, Platings, Shields, Stevens, Towler and Wood2020).

Figure 4. Comparison of the Rietveld-refined (red) and VASP-optimized (blue) structures of cation 2 in butenafine hydrochloride from the first (incorrect) refinement. The rms Cartesian displacement is 0.196 Å. Image generated using Mercury (Macrae et al., Reference Macrae, Sovago, Cottrell, Galek, McCabe, Pidcock, Platings, Shields, Stevens, Towler and Wood2020).

Figure 5. The Rietveld plot for the final refinement of butenafine hydrochloride. The blue crosses represent the observed data points, and the green line is the calculated pattern. The cyan curve is the normalized error plot. The red curve indicates the background. The vertical scale has been multiplied by a factor of 20× for 2θ > 9.0°. The row of blue tick marks indicates the calculated reflection positions.
Rietveld refinement was carried out using GSAS-II (Toby and Von Dreele, Reference Toby and Von Dreele2013). Only the 1.5–20.0° portion of the pattern was included in the refinement (d min = 1.321 Å). The y-coordinate of N1 was fixed to define the origin. All non-H bond distances and angles were subjected to restraints, based on a Mercury/Mogul Geometry Check (Bruno et al., Reference Bruno, Cole, Kessler, Luo, Motherwell, Purkis, Smith, Taylor, Cooper, Harris and Orpen2004; Sykes et al., Reference Sykes, McCabe, Allen, Battle, Bruno and Wood2011). The Mogul average and standard deviation for each quantity were used as the restraint parameters. The restraints contributed 1.9% to the final χ 2. The hydrogen atoms were included in calculated positions, which were recalculated during the refinement using Materials Studio (Dassault, 2021). The U iso of the heavy atoms were grouped by chemical similarity. The U iso for the H atoms were fixed at 1.2× the U iso of the heavy atoms to which they are attached. The peak profiles were described using the generalized microstrain model. The background was modeled using a 6-term shifted Chebyshev polynomial, and a peak at 5.42° 2θ to model the scattering from the Kapton capillary and any amorphous component.
The final refinement of 168 variables using 18,530 observations and 126 restraints yielded the residuals R wp = 0.0814 and GOF = 1.45. The largest peak (0.12 Å from Cl103) and hole (2.12 Å from C73) in the difference Fourier map were 0.20(4) and −0.16(4) eÅ−3, respectively. The largest errors in the difference plot (Figure 5) are in the shapes of some of the low-angle peaks.
The crystal structure was optimized using VASP (Kresse and Furthmüller, Reference Kresse and Furthmüller1996) (fixed experimental unit cell) through the MedeA graphical interface (Materials Design, 2016). The calculation was carried out on 16 2.4 GHz processors (each with 4 GB RAM) of a 64-processor HP Proliant DL580 Generation 7 Linux cluster at North Central College. The calculation used the GGA-PBE functional, a plane wave cutoff energy of 400.0 eV, and a k-point spacing of 0.5 Å−1 leading to a 1 × 2 × 1 mesh, and took ~18.5 h. A single-point density functional calculation (fixed experimental cell) and population analysis were carried out using CRYSTAL17 (Dovesi et al., Reference Dovesi, Erba, Orlando, Zicovich-Wilson, Civalleri, Maschio, Rerat, Casassa, Baima, Salustro and Kirtman2018). The basis sets for the H, C, N, and O atoms in the calculation were those of Gatti et al. (Reference Gatti, Saunders and Roetti1994), and that for Cl was that of Peintinger et al. (Reference Peintinger, Vilela Oliveira and Bredow2013). The calculations were run on a 3.5 GHz PC using 8 k-points and the B3LYP functional, and took ~7.5 h. A Hirshfeld surface analysis was caried out using CrystalExplorer17 (Turner et al., Reference Turner, McKinnon, Wolff, Grimwood, Spackman, Jayatilaka and Spackman2017).
III. RESULTS AND DISCUSSION
Butenafine hydrochloride occurs as a racemic co-crystal of equal numbers of the R- and S-enantiomers of the protonated butenafine cation. The rms Cartesian displacements between the Rietveld-refined and DFT-optimized structures of cations 1 and 2 are 0.068 and 0.122 Å (Figures 6 and 7), respectively. The excellent agreement is strong evidence that the structure is correct (van de Streek and Neumann, Reference van de Streek and Neumann2014). This discussion concentrates on the DFT-optimized structure. The asymmetric unit (with atom numbering) is illustrated in Figure 8. The displacement coefficients of the atoms in the t-butyl groups of both cations are larger than those of the other atoms, suggesting the possibility of disorder. Since the DFT calculation required an ordered model, we did not attempt to model any disorder. The crystal structure (Figure 9) is characterized by parallel stacks of aromatic rings along the b-axis. The approximate planes of the naphthalene rings are (−1 2 2) and (1 2 2), and those of the phenyl rings are (−3 3 2) and (1 1 −1).

Figure 6. Comparison of the Rietveld-refined (red) and VASP-optimized (blue) structures of cation 1 in butenafine hydrochloride from the final refinement. The rms Cartesian displacement is 0.068 Å. Image generated using Mercury (Macrae et al., Reference Macrae, Sovago, Cottrell, Galek, McCabe, Pidcock, Platings, Shields, Stevens, Towler and Wood2020).
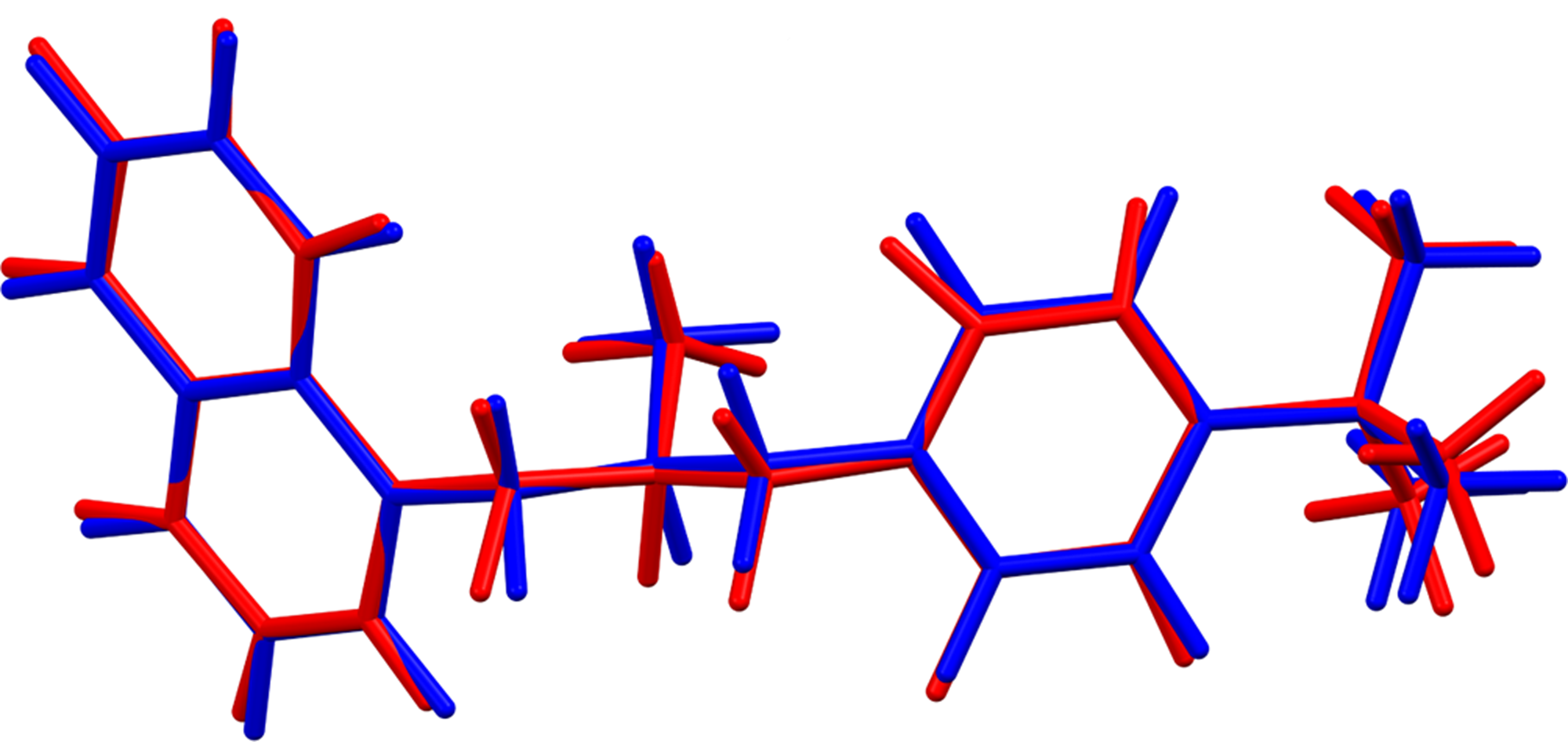
Figure 7. Comparison of the Rietveld-refined (red) and VASP-optimized (blue) structures of cation 2 in butenafine hydrochloride from the final refinement. The rms Cartesian displacement is 0.122 Å. Image generated using Mercury (Macrae et al., Reference Macrae, Sovago, Cottrell, Galek, McCabe, Pidcock, Platings, Shields, Stevens, Towler and Wood2020).
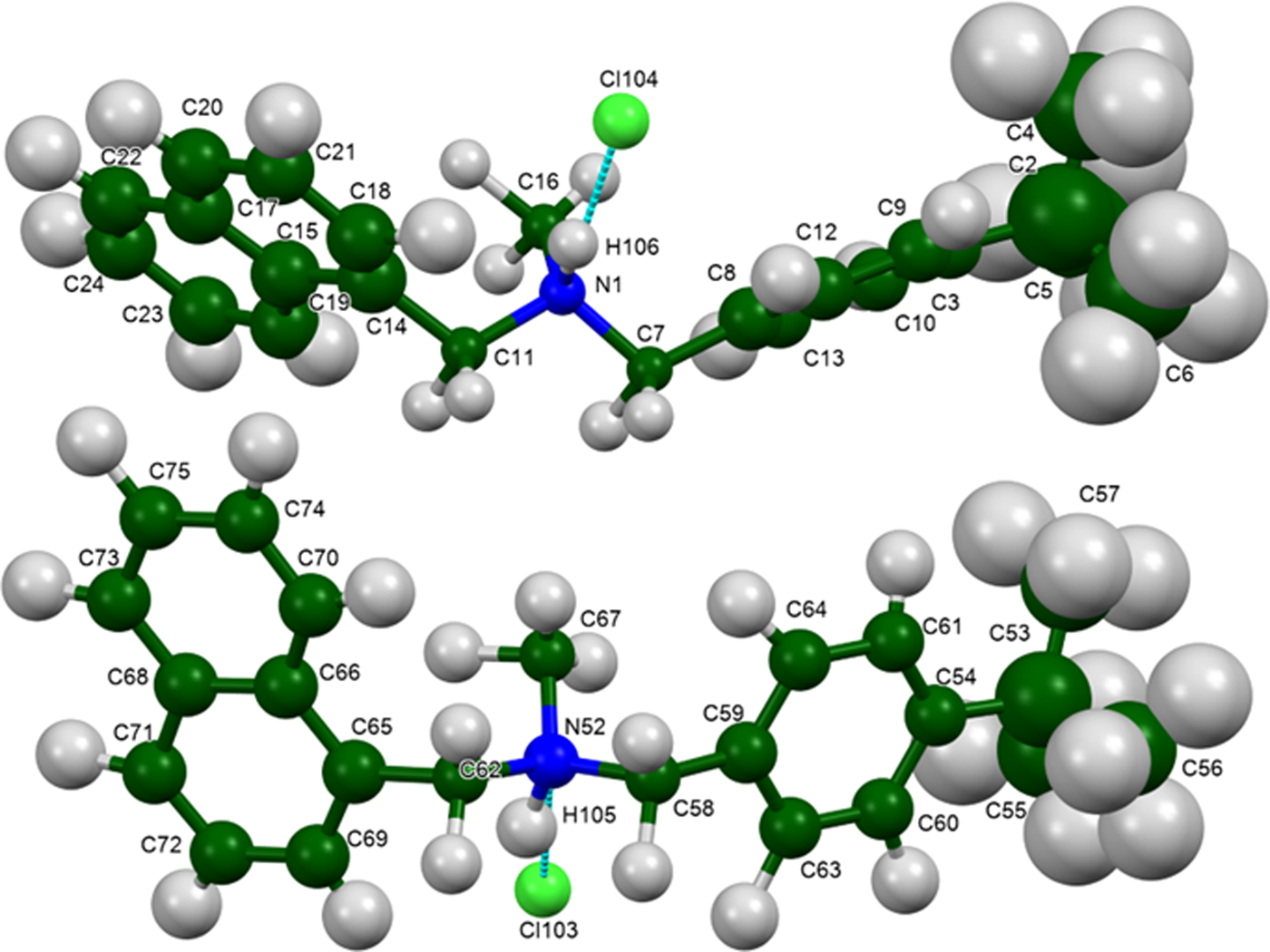
Figure 8. The asymmetric unit of butenafine hydrochloride, with the atom numbering. The atoms are represented by 50% probability spheroids/ellipsoids. Image generated using Mercury (Macrae et al., Reference Macrae, Sovago, Cottrell, Galek, McCabe, Pidcock, Platings, Shields, Stevens, Towler and Wood2020).

Figure 9. The crystal structure of butenafine hydrochloride, viewed down the a-axis. Image generated using Diamond (Crystal Impact, 2022).
All of the bond distances, bond angles, and torsion angles fall within the normal ranges indicated by a Mercury/Mogul Geometry check (Macrae et al., Reference Macrae, Sovago, Cottrell, Galek, McCabe, Pidcock, Platings, Shields, Stevens, Towler and Wood2020). Quantum chemical geometry optimization of the butenafine cations (DFT/B3LYP/6-31G*/water) using Spartan ‘18 (Wavefunction, 2020) indicated that (as expected) the two cations are identical in energy. A conformational analysis (MMFF force field) indicates that the global minimum-energy conformation curls on itself to form an intramolecular (naphthalene) C–H⋯phenyl hydrogen bond. Cation–anion and cation–cation interactions are thus important in determining the solid-state conformation.
Analysis of the contributions to the total crystal energy of the structure using the Forcite module of Materials Studio (Dassault, 2021) suggests that the intramolecular deformation energy is dominated by bond and angle distortion terms, as might be expected for fused ring systems. The intermolecular energy is dominated by electrostatic attractions, which in this force field analysis include hydrogen bonds. The hydrogen bonds are better analyzed using the results of the DFT calculation.
As expected, each cation forms a strong discrete N–H⋯Cl hydrogen bond (Table I). The chloride anions also act as acceptors in several C–H⋯Cl hydrogen bonds from methylene, methyl, and aromatic groups.
TABLE I. Hydrogen bonds (CRYSTAL17) in butenafine hydrochloride.
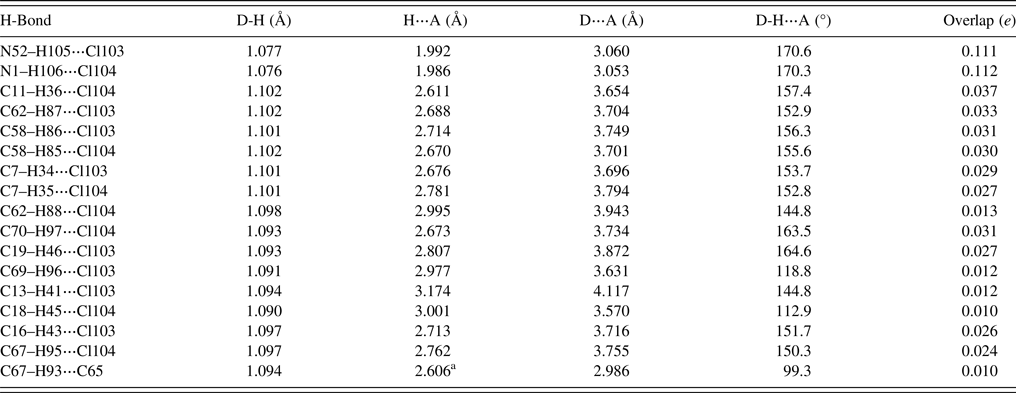
a Intramolecular.
The volume enclosed by the Hirshfeld surface of butenafine hydrochloride (Figure 10, Hirshfeld, Reference Hirshfeld1977; Turner et al., Reference Turner, McKinnon, Wolff, Grimwood, Spackman, Jayatilaka and Spackman2017) is 1033.08 Å3, 99.01% of ½ the unit cell volume. The packing density is thus higher than normal. The only significant close contacts (red in Figure 10) involve the hydrogen bonds. The volume/non-hydrogen atom is larger than normal at 20.9 Å3.

Figure 10. The Hirshfeld surface of butenafine hydrochloride. Intermolecular contacts longer than the sums of the van der Waals radii are colored blue, and contacts shorter than the sums of the radii are colored red. Contacts equal to the sums of radii are white. Image generated using CrystalExplorer (Turner et al., Reference Turner, McKinnon, Wolff, Grimwood, Spackman, Jayatilaka and Spackman2017).
The Bravais–Friedel–Donnay–Harker (Bravais, Reference Bravais1866; Friedel, Reference Friedel1907; Donnay and Harker, Reference Donnay and Harker1937) morphology suggests that we might expect an elongated morphology for butenafine hydrochloride, with 〈010〉 as the principal axis. A second-order spherical harmonic preferred orientation model was included in the refinement. The texture index was 1.007(0), indicating that preferred orientation was not significant for this rotated capillary specimen. The powder pattern of butenafine hydrochloride from this synchrotron data set has been submitted to ICDD for inclusion in the Powder Diffraction File.
IV. DEPOSITED DATA
The Crystallographic Information Framework (CIF) files containing the results of the Rietveld refinement (including the raw data) and the DFT geometry optimization were deposited with the ICDD. The data can be requested at [email protected].
ACKNOWLEDGMENTS
The use of the Advanced Photon Source at Argonne National Laboratory was supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract No. DE-AC02-06CH11357. This work was partially supported by the International Centre for Diffraction Data. We thank Lynn Ribaud and Saul Lapidus for their assistance in the data collection.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to declare.














