No CrossRef data available.
Article contents
Cation reducibility of LaNi0.5Ti0.5O3, LaNi0.5Ti0.45Co0.05O3, and LaNi0.45Co0.05Ti0.5O3 perovskites from X-ray powder diffraction data using the Rietveld method
Published online by Cambridge University Press: 11 May 2022
Abstract
In the present work, LaNi0.5Ti0.5O3, LaNi0.5Ti0.45Co0.05O3, and LaNi0.45Co0.05Ti0.5O3 perovskites were synthesized using the modified Pechini method. After reduction, the studied perovskites changed crystal structure from the perovskite crystal structure to a cubic symmetry, with space group 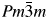 $Pm\bar{3}m$. The reduction partially decomposed the samples to Ni0 (Co free perovskite), Ni0–Co0, La2O3, La2TiO5, and non-stoichiometric La2NiO4, depending on H2 content of the reductive gases. The degree of reduction of nickel from LaNi0.5Ti0.5O3 reduced with 1.8% H2/Ar and 10% H2/Ar was equal to 36.5% and 95.3%, respectively, while that from LaNi0.5Ti0.45Co0.05O3 or LaNi0.45Co0.05Ti0.5O3, including cobalt, reduced with 10% H2/Ar, was equal to 71.9% and 93.9%, respectively. LaNi0.5Ti0.45Co0.05O3 showed Ni3+ and Co3+ amounts higher than the other perovskites. By increasing H2 content in the reductive mixture from 1.8% to 10%, sintering of metallic nickel was not observed. Moreover, Ni0 displayed weaker metal–support interaction than that observed for Co0, where the support was composed of La containing oxides. LaNi0.5Ti0.5O3 perovskite was used as a catalyst for steam reforming of methane. Syngas production was attributed to the number of Ni sites determined using Rietveld Refinement of X-ray diffraction pattern of this catalyst after the reaction.
$Pm\bar{3}m$. The reduction partially decomposed the samples to Ni0 (Co free perovskite), Ni0–Co0, La2O3, La2TiO5, and non-stoichiometric La2NiO4, depending on H2 content of the reductive gases. The degree of reduction of nickel from LaNi0.5Ti0.5O3 reduced with 1.8% H2/Ar and 10% H2/Ar was equal to 36.5% and 95.3%, respectively, while that from LaNi0.5Ti0.45Co0.05O3 or LaNi0.45Co0.05Ti0.5O3, including cobalt, reduced with 10% H2/Ar, was equal to 71.9% and 93.9%, respectively. LaNi0.5Ti0.45Co0.05O3 showed Ni3+ and Co3+ amounts higher than the other perovskites. By increasing H2 content in the reductive mixture from 1.8% to 10%, sintering of metallic nickel was not observed. Moreover, Ni0 displayed weaker metal–support interaction than that observed for Co0, where the support was composed of La containing oxides. LaNi0.5Ti0.5O3 perovskite was used as a catalyst for steam reforming of methane. Syngas production was attributed to the number of Ni sites determined using Rietveld Refinement of X-ray diffraction pattern of this catalyst after the reaction.
- Type
- Technical Article
- Information
- Copyright
- Copyright © The Author(s), 2022. Published by Cambridge University Press on behalf of International Centre for Diffraction Data



