Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Yonezaki, Yoshinori
and
Takei, Shino
2016.
Photochromism and emission-color change in Ba3MgSi2O8-based phosphors.
Journal of Luminescence,
Vol. 173,
Issue. ,
p.
237.
Yonezaki, Yoshinori
Takei, Shino
and
Ogawa, Kazuya
2017.
Room-temperature emission-color switching of Ba 3 MgSi 2 O 8 : Eu 2+ and the photochemical reaction mechanism.
Journal of Luminescence,
Vol. 188,
Issue. ,
p.
12.
Yonezaki, Yoshinori
2018.
Structural influence on photochromic behaviors of Eu 2+ -doped glaserite-type silicates.
Journal of Luminescence,
Vol. 195,
Issue. ,
p.
408.
Yonezaki, Yoshinori
Takei, Shino
and
Matsumoto, Sayaka
2018.
Emission-color change caused by photoredox reactions of europium ions in Ba3MgSi2O8.
Journal of Photochemistry and Photobiology A: Chemistry,
Vol. 367,
Issue. ,
p.
406.
Avdeev, Maxim
Xia, Qingbo
Sale, Matthew
Allison, Morgan
and
Ling, Chris D.
2018.
Crystal structure and monoclinic distortion of glaserite-type Ba3MnSi2O8.
Journal of Solid State Chemistry,
Vol. 266,
Issue. ,
p.
1.
Yonezaki, Yoshinori
2020.
Emission-color change in Eu-doped high-symmetry glaserite-type silicates.
Journal of Photochemistry and Photobiology A: Chemistry,
Vol. 398,
Issue. ,
p.
112645.
Fernández-Rodríguez, L.
Allix, M.
Gorni, G.
Canizarès, A.
Ory, S.
Mather, G.C.
Durán, A.
Levy, D.
and
Pascual, M.J.
2022.
Persistent luminescence of Eu/Dy-doped Sr2MgSi2O7 glass-ceramics processed by aerodynamic levitation.
Journal of the European Ceramic Society,
Vol. 42,
Issue. 16,
p.
7596.
SATO, Yasushi
KUMASHIRO, Chihiro
OKIMOTO, Kokoro
TOMITA, Koji
and
KAKIHANA, Masato
2022.
Synthesis and photoluminescence properties of Ca<sub>3</sub>MgSi<sub>2</sub>O<sub>8</sub> with high Eu<sup>2+</sup> concentration.
Journal of the Ceramic Society of Japan,
Vol. 130,
Issue. 1,
p.
49.
Yonezaki, Yoshinori
2022.
CuKα-radiation (λ = 0.154 nm) induced emission-color change in Eu-doped KNaSO4.
Sensors and Actuators A: Physical,
Vol. 338,
Issue. ,
p.
113494.
Kahlenberg, Volker
Prosser, Lukas
Salzmann, Michael F.
and
Hejny, Clivia
2022.
On the incorporation of strontium into the crystal structure of bredigite: structural effects and phase transition.
Mineralogy and Petrology,
Vol. 116,
Issue. 2,
p.
151.
Yonezaki, Yoshinori
and
Katsumata, Yuki
2024.
Structural consideration on Eu2+ distribution inside glaserite-type Na(Ba, Sr)PO4: Eu2+ phosphor: High-intensity and broadband emission consisting of three overlapping Eu2+ emission bands.
Optical Materials,
Vol. 157,
Issue. ,
p.
116185.
Yang, Jiaqing
Lei, Peng
Song, Xiaoqiang
Yin, Changzhi
Zhang, Meng
Lei, Weicheng
Cai, Yiyang
Cheng, Mingfei
Liu, Yaodong
Chen, Zihang
Hu, Yaqing
Lu, Wenzhong
and
Lei, Wen
2025.
Abnormal dielectric behavior of glaserite-type Ba
3−
x
Sr
x
MgSi
2O
8solid solution
.
Journal of Advanced Ceramics,
Vol. 14,
Issue. 2,
p.
9221020.
Lu, Juan
Tang, Wan-Chi
Yang, Cheng-Fu
and
Chang, Jui-Yang
2025.
Effect of different BaO contents on the photoluminescence properties of the Eu2+-doped high thermal stability Sr3−xBaxMgSi2O8 phosphors.
International Journal of Modern Physics B,
Vol. 39,
Issue. 06,
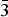 ${\bar 3}$m1 (Z = 1), and P
${\bar 3}$m1 (Z = 1), and P ${\bar 3}$ (Z = 3). They originate in SiO4 tilting caused by size mismatch between alkali–earth cations and their site spaces. For x ≤ 0.5, SiO4 tilting occur every other interlayer space, whereas for x ≥ 2.5, all the SiO4 tilt.
${\bar 3}$ (Z = 3). They originate in SiO4 tilting caused by size mismatch between alkali–earth cations and their site spaces. For x ≤ 0.5, SiO4 tilting occur every other interlayer space, whereas for x ≥ 2.5, all the SiO4 tilt.


