Article contents
Ultrastructural characterization of host–parasite interactions of Plasmodium coatneyi in rhesus macaques
Published online by Cambridge University Press: 04 October 2021
Abstract
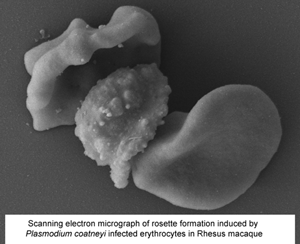
Plasmodium coatneyi has been proposed as an animal model for human Plasmodium falciparum malaria as it appears to replicate many aspects of pathogenesis and clinical symptomology. As part of the ongoing evaluation of the rhesus macaque model of severe malaria, a detailed ultrastructural analysis of the interaction between the parasite and both the host erythrocytes and the microvasculature was undertaken. Tissue (brain, heart and kidney) from splenectomized rhesus macaques and blood from spleen-intact animals infected with P. coatneyi were examined by electron microscopy. In all three tissues, similar interactions (sequestration) between infected red blood cells (iRBC) and blood vessels were observed with evidence of rosette and auto-agglutinate formation. The iRBCs possessed caveolae similar to P. vivax and knob-like structures similar to P. falciparum. However, the knobs often appeared incompletely formed in the splenectomized animals in contrast to the intact knobs exhibited by spleen intact animals. Plasmodium coatneyi infection in the monkey replicates many of the ultrastructural features particularly associated with P. falciparum in humans and as such supports its use as a suitable animal model. However, the possible effect on host–parasite interactions and the pathogenesis of disease due to the use of splenectomized animals needs to be taken into consideration.
- Type
- Research Article
- Information
- Creative Commons
- This is a work of the US Government and is not subject to copyright protection within the United States. Published by Cambridge University Press.
- Copyright
- Copyright © United States Army - Armed Forces Research Institute of Medical Sciences (AFRIMS), 2021
References
- 1
- Cited by



