Article contents
Trypanosoma rangeli infection increases the exposure and predation endured by Rhodnius prolixus
Published online by Cambridge University Press: 28 September 2021
Abstract
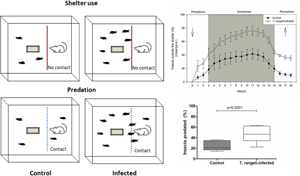
Trypanosoma rangeli is a protozoan that infects triatomines and mammals in Latin America, sharing hosts with Trypanosoma cruzi, the etiological agent of Chagas disease. Trypanosoma rangeli does not cause disease to humans but is strongly pathogenic to its invertebrate hosts, increasing mortality rates and affecting bug development and reproductive success. We have previously shown that this parasite is also capable of inducing a general increase in the locomotory activity of its vector Rhodnius prolixus in the absence of host cues. In this work, we have evaluated whether infection impacts the insect–vertebrate host interaction. For this, T. rangeli-infected and uninfected R. prolixus nymphs were released in glass arenas offering single shelters. After a 3-day acclimatization, a caged mouse was introduced in each arena and shelter use and predation rates were evaluated. Trypanosoma rangeli infection affected all parameters analysed. A larger number of infected bugs was found outside shelters, both in the absence and presence of a host. Infected bugs also endured greater predation rates, probably because of an increased number of individuals that attempted to feed. Interestingly, mice that predated on infected bugs did not develop T. rangeli infection, suggesting that the oral route is not effective for these parasites, at least in our system. Finally, a smaller number of infected bugs succeeded in feeding in this context. We suggest that, although T. rangeli is not transmitted orally, an increase in the proportion of foraging individuals would promote greater parasite transmission rates through an increased frequency of very effective infected-bug bites.
- Type
- Research Article
- Information
- Copyright
- Copyright © The Author(s), 2021. Published by Cambridge University Press
References
- 7
- Cited by



