Published online by Cambridge University Press: 29 March 2021
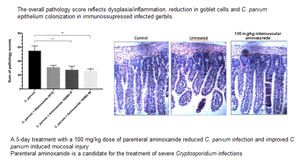
Cryptosporidiosis is a gastrointestinal illness with profuse diarrhoea. Although there are no other Food and Drug Administration (FDA)-approved alternatives for the treatment of cryptosporidiosis, nitazoxanide (NTZ) can be qualified as partially effective. In immunosuppressed conditions, severe and/or disseminated cryptosporidiosis may occur and patients should be treated parenterally. To achieve the goal of developing parenteral treatment for cryptosporidiosis, the current study was undertaken to investigate the in vitro and in vivo anticryptosporidial activity of aminoxanide. This new l-tert-leucyl thiazolide is a soluble prodrug of tizoxanide (TIZ), the main metabolite of NTZ. Confirming the good efficacy of aminoxanide in Cryptosporidium parvum-infected HCT-8 cells with a 50% inhibitory concentration of 1.55 μm (±0.21), in immunosuppressed C. parvum-infected Mongolian gerbils (Meriones unguiculatus), a 5-day treatment with a daily intramuscular dose of 100 mg kg−1 aminoxanide resulted in a 72.5% oocyst excretion inhibition, statistically equivalent to 75.5% in gerbils treated with a 4-fold lower oral dose of NTZ. Cryptosporidium parvum-induced intestinal pathology and inflammation were improved. Aminoxanide provides an injectable form of TIZ that NTZ was unable to do and is a promising drug for which optimization of the formulation should be further explored. These results represent a first promising step towards the goal of developing a parenteral treatment for cryptosporidiosis.