Introduction
The occurrence of surface glycoconjugates in parasitic protozoa is of paramount importance since they are crucially involved in processes such as immune evasion, host cell invasion and endocytosis. Most parasitic protozoa undergo complex life cycles and must adapt to changing hosts and environments. Surface glycans constitute a protective barrier that contributes to adaptation and the establishment of infection by participating in the subversion of the immune response and in specific interactions with host surface molecules. Thus, specific sugar–lectin interactions are involved in the colonization of the gut of the insect vector as well as in mammalian host cell recognition and parasite internalization. In this latter process, specific glycan–lectin interactions mediate mammalian host cell recognition and parasite uptake. Pattern recognition receptors (PRRs) present on the surface of immune cells distinguish pathogen-associated molecular patterns (PAMPs). The receptors used for parasite infection vary and include complement receptors, scavenger receptors, Toll-like receptors (TLR) and mannose receptors. The understanding of these interactions will provide an insight into how protozoa implement infection and subvert the host immune response.
In relation to the immune response, several carbohydrate-binding proteins, either expressed on the surface of cells of the immune system or released, play an essential role in the control of innate and adaptive immunity. These include C-type lectin receptors, sialic acid-binding immunoglobulin (Ig)-like lectins (siglecs) and galectins that interact with distinct glycan structures (van Kooyk and Rabinovich, Reference van Kooyk and Rabinovich2008). DC-SIGN, a C-type lectin receptor found on the surface of dendritic cells specifically binds mannose and/or fucose-terminated glycans. Siglecs are included in the group of Ig-type (I-type) lectins and interact with a wide variety of structurally distinct carbohydrate ligands (Pillai et al., Reference Pillai, Netravali, Cariappa and Mattoo2012). Galectins are a family of soluble lectins that bind β-galactose (β-Gal)-containing glycoconjugates such as glycans containing N-acetyllactosamine and are thought to be able to associate with host membrane glycans to form a cell-surface network for an optimal receptor spacing and signalling (Liu and Rabinovich, Reference Liu and Rabinovich2005; Nieminen et al., Reference Nieminen, Kuno, Hirabayashi and Sato2007). While they can act as effector factors, inhibiting pathogen adhesion and entry or stimulating phagocytosis, parasites can also make use of host galectins to facilitate host cell invasion. Furthermore, in the case of secreted glycoproteins, such as cytokines, chemokines and antibodies, the sugar portion has been described to perform important functions. This is the case of the N-glycans attached to the Fc portion of IgG, that when sialylated send an inhibitory signal to the immune system (Kaneko et al., Reference Kaneko, Nimmerjahn and Ravetch2006; Anthony et al., Reference Anthony, Kobayashi, Wermeling and Ravetch2011). Glycans are also central to lymphocyte development (Stanley and Okajima, Reference Stanley and Okajima2010) and leucocyte homing (Lowe, Reference Lowe2003; Mitoma et al., Reference Mitoma, Bao, Petryanik, Schaerli, Gauguet, Yu, Kawashima, Saito, Ohtsubo, Marth, Khoo, von Andrian, Lowe and Fukuda2007). Finally, an additional immunomodulatory pathway in which surface glycans have a major role is the lectin pathway (LP) for complement activation, which requires the mannose-binding lectin and ficolins, rather than the standard components (Matsushita, Reference Matsushita2010) necessary for the activation of the classical and alternative pathways (AP). The identification of singular aspects related to glycan composition in kinetoplastids and their interaction with host lectins may unveil opportunities for drug design using agents that specifically bind to carbohydrate moieties important for parasite survival within the mammalian host.
The nature of surface glycans in Trypanosoma cruzi
As in the case of other protozoan parasites of medical and veterinary relevance, the surface of T. cruzi is heavily glycosylated. The dense glycocalyx performs specific and significant functions such as protection against the host defence mechanisms and/or the interaction with changing environments (Noireau et al., Reference Noireau, Diosque and Jansen2009; Romano et al., Reference Romano, Cueto, Casassa, Vanrell, Gottlieb and Colombo2012). The carbohydrate nature of the surface coat strongly depends on the life stage and differentiation involves unique changes in its composition (de Lederkremer and Agusti, Reference de Lederkremer and Agusti2009).
The most abundant components of the T. cruzi surface coat, especially in the epimastigote form, are glycosylphosphatidylinositol (GPI)-anchored glycoconjugates of varied nature (Ferguson, Reference Ferguson1999). The structure of this coat has been described as a basal layer of glycoinositolphospholipids (GIPLs) and phospholipid (Previato et al., Reference Previato, Gorin, Mazurek, Xavier, Fournet, Wieruszesk and Mendonca-Previato1990; de Lederkremer et al., Reference de Lederkremer, Lima, Ramirez, Ferguson, Homans and Thomas-Oates1991; Carreira et al., Reference Carreira, Jones, Wait, Previato and Mendonca-Previato1996) from which other GPI-anchored molecules protrude (Previato et al., Reference Previato, Wait, Jones, DosReis, Todeschini, Heise and Previato2004). The major species are mucin-like proteins (Pereira-Chioccola et al., Reference Pereira-Chioccola, Acosta-Serrano, Correia de Almeida, Ferguson, Souto-Padron, Rodrigues, Travassos and Schenkman2000; Buscaglia et al., Reference Buscaglia, Campo, Frasch and Di Noia2006), which are heavily O-glycosylated, while the less abundant include trans-sialidase (TS) (Previato et al., Reference Previato, Andrade, Pessolani and Mendonca-Previato1985; Schenkman and Eichinger, Reference Schenkman and Eichinger1993), mucin-associated proteins (MASPs) (dos Santos et al., Reference dos Santos, Freitas, Lobo, Rodrigues-Luiz, Mendes, Oliveira, Andrade, Chiari, Gazzinelli, Teixeira, Fujiwara and Bartholomeu2012), Gp85 surface glycoproteins (Mattos et al., Reference Mattos, Tonelli, Colli and Alves2014), trypomastigote small surface antigen (TSSA) (Canepa et al., Reference Canepa, Degese, Budu, Garcia and Buscaglia2012) and Toll-T antigens (Quanquin et al., Reference Quanquin, Galaviz, Fouts, Wrightsman and Manning1999). Recent studies support the idea that lipid-based domains, and particularly lipid rafts, are responsible for the fine organization of all these components (Mucci et al., Reference Mucci, Lantos, Buscaglia, Leguizamon and Campetella2017).
GIPL was the first glycoconjugate characterized in T. cruzi and can be found as a free entity or anchored to proteins. GIPLs were originally defined as lipopeptidophosphoglycans (LPPGs) because of the amino acids present in the early preparations (De Lederkremer et al., Reference De Lederkremer, Alves, Fonseca and Colli1976). However, with the solution of their structure, it was established that these LPPGs are typical GPIs (Previato et al., Reference Previato, Gorin, Mazurek, Xavier, Fournet, Wieruszesk and Mendonca-Previato1990; de Lederkremer et al., Reference de Lederkremer, Lima, Ramirez, Ferguson, Homans and Thomas-Oates1991). The core of GIPLs is constituted by Manα(1,2)-Manα(1,6)-Manα(1,4)-GlcNα(1,6)-myo-inositol-PO4-lipid, in some cases with four mannose residues, where the lipid moiety is either 1-O-hexadecyl-2-O-palmitoyl glycerol or ceramide (McConville and Ferguson, Reference McConville and Ferguson1993). Galactofuranose (Galf) and aminoethylphosphonic acid are substituents that can be found attached to different positions of the main core, conferring a certain microheterogeneity to the oligosaccharide structure (McConville and Ferguson, Reference McConville and Ferguson1993).
Mucins are the most abundant glycoproteins in the T. cruzi surface membrane. They are a complex and heterogeneous group of variable proteins constituted by a polypeptidic core of 50–200 amino acids, rich in serine and threonine residues many of which are O-glycosylated (Buscaglia et al., Reference Buscaglia, Campo, Frasch and Di Noia2006). Mucins are O-glycosylated with N-acetylglucosamine (GlcNAc), which is rather unique since the glycosyltransferases that catalyse this transfer in other organisms usually use UDP-N-acetylgalactosamine as a precursor (Previato et al., Reference Previato, Jones, Xavier, Wait, Travassos, Parodi and Mendonca-Previato1995, Reference Previato, Sola-Penna, Agrellos, Jones, Oeltmann, Travassos and Mendonca-Previato1998). The O-linked GlcNAc residues can be further elongated or remain unsubstituted. Galactose is present in all mucin oligosaccharide elongations in either the pyranosic (Galp) or Galf configuration (Acosta-Serrano et al., Reference Acosta-Serrano, Almeida, Freitas-Junior, Yoshida and Schenkman2001). Terminal β-Galp residues can be further branched with sialic acid acquired from the host through TSs present on the membrane surface (Previato et al., Reference Previato, Jones, Goncalves, Wait, Travassos and Mendonca-Previato1994; Serrano et al., Reference Serrano, Schenkman, Yoshida, Mehlert, Richardson and Ferguson1995).
TSs are another group of GPI-anchored proteins found on the surface of T. cruzi, and their activity allows the parasite to bypass its lack of de novo synthesis of sialic acid, that is instead salvaged from the host (Previato et al., Reference Previato, Andrade, Pessolani and Mendonca-Previato1985). TSs catalyse the transfer of sialic acid from an α(2,3)-linkage in the donor to a terminal β-Galp acceptor of the parasite mucins (Schenkman et al., Reference Schenkman, Ferguson, Heise, de Almeida, Mortara and Yoshida1993). It has also been shown that T. cruzi TS (TcTS) can efficiently transfer α(2,3)-linked N-glycolylneuraminic acid (Neu5Gc) to terminal β-Gal groups (Agusti et al., Reference Agusti, Giorgi and de Lederkremer2007; Schroven et al., Reference Schroven, Meinke, Ziegelmuller and Thiem2007). This specific activity of TcTS is unique because of several aspects. First, TcTSs, unlike mammalian TSs, do not use cytidine monophospho (CMP)-sialic acid as the monosaccharide donor. Additionally, they appear to be located on the parasite surface and not in the Golgi apparatus, which is where they carry out their normal function in other organisms. Finally, unlike conventional sialidases, TcTSs are more efficient in transferring terminal sialic acids between glycoconjugates rather than hydrolysing them. Recent studies have also shown that sialylated mucins are present in membrane lipid-rafts far away from TS and that the sialylation process is performed by microvesicles associated with active TcTS (Lantos et al., Reference Lantos, Carlevaro, Araoz, Ruiz Diaz, Camara Mde, Buscaglia, Bossi, Yu, Chen, Bertozzi, Mucci and Campetella2016).
Other GPI-anchored proteins are the Gp85 surface glycoproteins, TSSAs and MASPs. Gp85 glycoproteins are usually included in the TS superfamily as TS-like proteins yet they lack TS activity (Buscaglia et al., Reference Buscaglia, Campo, Frasch and Di Noia2006) and appear to be involved in host–parasite interactions (Alves and Colli, Reference Alves and Colli2008). TSSAs are polymorphic mucin-like molecules with a conserved hydrophobic C-terminus compatible with the GPI-anchoring signal, and a variable central region responsible for their antigenicity (Di Noia et al., Reference Di Noia, Buscaglia, De Marchi, Almeida and Frasch2002). Finally, MASPs are GPI-anchored proteins that have been found predominantly in the proteome of trypomastigotes (Atwood et al., Reference Atwood, Weatherly, Minning, Bundy, Cavola, Opperdoes, Orlando and Tarleton2005). Like mucins, they contain highly conserved N- and C-terminal domains plus a variable central region (Bartholomeu et al., Reference Bartholomeu, Cerqueira, Leao, daRocha, Pais, Macedo, Djikeng, Teixeira and El-Sayed2009) yet they appear to be N-glycosylated (Atwood et al., Reference Atwood, Minning, Ludolf, Nuccio, Weatherly, Alvarez-Manilla, Tarleton and Orlando2006).
Glycans and immunomodulation during T. cruzi infection
The complement is the first line of defence of the innate immune system against invading microbes. Trypanosoma cruzi invasion generates an immediate immune response due to the interaction of the parasite with complement molecules. It has been shown that the complement can be activated by all T. cruzi forms: amastigote (Iida et al., Reference Iida, Whitlow and Nussenzweig1989), epimastigote (Nogueira et al., Reference Nogueira, Bianco and Cohn1975) and trypomastigote (Kipnis et al., Reference Kipnis, Krettli and Dias da Silva1985), but only the non-infective epimastigotes are susceptible to complement lysis. During the first seconds after T. cruzi infection, signal glycoproteins on the parasite surface can interact with host PRRs such as mannose-binding lectins and ficolins and lead to the activation of the LP and AP (Fig. 1) (Cestari et al., Reference Cestari, Evans-Osses, Schlapbach, de Messias-Reason and Ramirez2013). However, T. cruzi parasites can undertake a series of strategies to escape the effects of both innate and adaptive immunity. There are at least three different mechanisms of complement system evasion by T. cruzi. One of such mechanisms is the translocation of calreticulin (TcCRT), a calcium binding protein normally expressed in the endoplasmic reticulum, to the surface membrane on the flagellar portion of the parasite (Ferreira et al., Reference Ferreira, Molina, Valck, Rojas, Aguilar, Ramirez, Schwaeble and Ferreira2004a, Reference Ferreira, Valck, Sanchez, Gingras, Tzima, Molina, Sim, Schwaeble and Ferreira2004b; Gonzalez et al., Reference Gonzalez, Valck, Sanchez, Hartel, Mansilla, Ramirez, Fernandez, Arias, Galanti and Ferreira2015). This translocation allows TcCRT to interact with mannose-binding lectins and ficolins and this way interfere with the normal activation of the LP and classical pathway and enhance the rate of the internalization of parasites by host cells (Fig. 1) (Gonzalez et al., Reference Gonzalez, Valck, Sanchez, Hartel, Mansilla, Ramirez, Fernandez, Arias, Galanti and Ferreira2015). Another escape mechanism from the innate immune response is the release of plasma membrane microvesicles by T. cruzi parasites. Extracellular vesicles contain several signal factors including glycoproteins and enzymes involved in carbohydrate metabolism, which also interfere with the LP and classical pathway activation (Geiger et al., Reference Geiger, Hirtz, Becue, Bellard, Centeno, Gargani, Rossignol, Cuny and Peltier2010; Ramirez et al., Reference Ramirez, Deolindo, de Messias-Reason, Arigi, Choi, Almeida and Evans-Osses2017).

Fig. 1. Scheme of the interplay between T. cruzi surface glycans and mammalian host cells. Upon infection, surface glycans within PAMPs can interact with host cell (i.e. myeloid and dendritic cells) PRRs and lead to the activation of the complement LP and AP. TcCRT translocates from the endoplasmic reticulum to the surface membrane in the zone of flagellum emergence and interacts with PRRs interfering in the normal activation of the complement LP and AP. Sialic acid (SIA) is transferred from the host cell membrane to parasite surface proteins such as mucins (TcMUC), conferring this way a molecular camouflage that hinders an effective immune response. The transfer of SIA is catalysed by TcTS and leads to an inhibition of the activation of T lymphocytes. In addition, sialylated mucins may interact with siglecs expressed on the surface of T cells and inhibit cytokine production.
One of the most important carbohydrates interfering with the immune response against T. cruzi infection is sialic acid. Trypanosoma cruzi transfers sialic acid from the host to its own surface glycoproteins creating this way a perfect molecular camouflage that hinders an effective immune response (Fig. 1) (Argibay et al., Reference Argibay, Di Noia, Hidalgo, Mocetti, Barbich, Lorenti, Bustos, Tambutti, Hyon, Frasch and Sanchez2002; Gao et al., Reference Gao, Wortis and Pereira2002; Freire-de-Lima et al., Reference Freire-de-Lima, Alisson-Silva, Carvalho, Takiya, Rodrigues, DosReis, Mendonca-Previato, Previato and Todeschini2010). In addition, sialylated mucins may interact with siglecs expressed on the T cell surface and inhibit clonal expansion and cytokine production by CD4+ lymphocytes (Nunes et al., Reference Nunes, Fortes, Silva-Filho, Terra-Granado, Santos, Conde, de Araujo Oliveira, Freire-de-Lima, Martins, Pinheiro, Takyia, Freire-de-Lima, Todeschini, Dosreis and Morrot2013). On the other hand, TcTS interferes with the activation of T lymphocytes (Fig. 1) (Pennock et al., Reference Pennock, White, Cross, Cheney, Tamburini and Kedl2013). This latter process involves the loss of sialic acid residues from O-linked oligosaccharides and the exposure of free Galβ(1,3) residues (Galvan et al., Reference Galvan, Murali-Krishna, Ming, Baum and Ahmed1998; Priatel et al., Reference Priatel, Chui, Hiraoka, Simmons, Richardson, Page, Fukuda, Varki and Marth2000).
Host cell invasion
Trypanosoma cruzi has a quite complex life cycle that involves an obligate intracellular stage for parasite duplication. Cell invasion involves a strict and complex interaction between the parasite and the host cell. The first step of this process is the adhesion of the parasite to the target cell which involves the recognition of molecules present on the surface of both parasite and host cells. Several molecules of T. cruzi surface are involved, among them glycoproteins of the Gp85/TS family, and mucins are of greatest interest. β-Gal residues on surface glycoproteins have been suggested to mediate parasite attachment and entry in dendritic (Vray et al., Reference Vray, Camby, Vercruysse, Mijatovic, Bovin, Ricciardi-Castagnoli, Kaltner, Salmon, Gabius and Kiss2004) and smooth muscle cells (Kleshchenko et al., Reference Kleshchenko, Moody, Furtak, Ochieng, Lima and Villalta2004; Vray et al., Reference Vray, Camby, Vercruysse, Mijatovic, Bovin, Ricciardi-Castagnoli, Kaltner, Salmon, Gabius and Kiss2004). In addition, cruzipain, a major cysteine peptidase has also a role in immune evasion, host cell invasion and intracellular development. After the binding and recognition of the parasite by the host cell surface, T. cruzi is internalized by two possible mechanisms: phagocytosis (Vieira et al., Reference Vieira, Dutra, Carvalho, Cunha-e-Silva, Souto-Padron and Souza2002) and endocytosis (Schenkman and Mortara, Reference Schenkman and Mortara1992). Once inside the host cell, parasites are confined in the parasitophorous vacuole, a membrane structure that protects them from lysosome attack while replicating. Probably one of the most important events of host cell invasion is the ‘escape’ from the parasitophorous vacuole. Also in this process surface glycoproteins have a major function (Andrews and Whitlow, Reference Andrews and Whitlow1989; Stecconi-Silva et al., Reference Stecconi-Silva, Andreoli and Mortara2003).
Surface glycans in Trypanosoma brucei
The T. brucei surface coat exhibits a dense layer of GPI-anchored glycoproteins, such as the variant surface glycoproteins (VSGs) or procyclin found in the bloodstream or procyclic forms of the parasite, respectively. In a minor amount, other glycosylated proteins are expressed in the surface membrane, such as the trans-membrane invariant surface glycoproteins (ISGs) (Ziegelbauer and Overath, Reference Ziegelbauer and Overath1992; Ziegelbauer et al., Reference Ziegelbauer, Multhaup and Overath1992), the transferrin receptor (TfR) (Grab et al., Reference Grab, Shaw, Wells, Verjee, Russo, Webster, Naessens and Fish1993) and the haptoglobin–haemoglobin receptor (Vanhollebeke et al., Reference Vanhollebeke, De Muylder, Nielsen, Pays, Tebabi, Dieu, Raes, Moestrup and Pays2008) which are both GPI-anchored and located in the flagellar pocket. In addition, it has been reported that epimastigote forms found in the salivary glands of the tsetse fly present a stage-specific coat of a GPI-anchored protein named bloodstream stage alanine-rich protein or Brucei alanine-rich protein (BARP) (Nolan et al., Reference Nolan, Jackson, Biggs, Brabazon, Pays, Van Laethem, Paturiaux-Hanocq, Elliott, Voorheis and Pays2000; Urwyler et al., Reference Urwyler, Studer, Renggli and Roditi2007). The differentiation of epimastigotes to metacyclic trypomastigote forms is associated with the loss of BARP and to the expression of a new coat of metacyclic VSGs (Tetley et al., Reference Tetley, Turner, Barry, Crowe and Vickerman1987; Ginger et al., Reference Ginger, Blundell, Lewis, Browitt, Gunzl and Barry2002) and of a small family of metacyclic invariant surface glycoproteins which protrude and remain accessible for antibody recognition (Casas-Sánchez et al., Reference Casas-Sánchez, Perally, Ramaswamy, Haines, Rose, Yunta, Aguilera-Flores, Lehane, Almeida, Boulanger and Acosta-Serrano2018). All these glycoproteins are mainly N-glycosylated with different structures containing oligomannose, paucimannose and complex-type glycans (Bangs et al., Reference Bangs, Doering, Englund and Hart1988; Zamze et al., Reference Zamze, Wooten, Ashford, Ferguson, Dwek and Rademacher1990; Strang et al., Reference Strang, Allen, Holder and van Halbeek1993; Treumann et al., Reference Treumann, Zitzmann, Hulsmeier, Prescott, Almond, Sheehan and Ferguson1997; Mehlert et al., Reference Mehlert, Richardson and Ferguson1998a, Reference Mehlert, Zitzmann, Richardson, Treumann and Ferguson1998b, Reference Mehlert, Wormald and Ferguson2012; Acosta-Serrano et al., Reference Acosta-Serrano, O'Rear, Quellhorst, Lee, Hwa, Krag and Englund2004).
Specifically, VSGs are homodimers susceptible to N-glycosylation with one, two or three N-linked oligosaccharides depending on the VSG class. Thus, in Class 1 VSGs only one asparagine is modified with triantennary oligomannose structures (Man9–5GlcNAc2); Class 2 VSGs have two N-glycosylation sites, one of them is occupied with oligomannose structures and the other with structure type Man4–3GlcNAc2 and biantennary complex glycans and Class 3 VSGs are modified with a combination of oligomannose and complex biantennary glycans (Zamze et al., Reference Zamze, Wooten, Ashford, Ferguson, Dwek and Rademacher1990, Reference Zamze, Ashford, Wooten, Rademacher and Dwek1991; Mehlert et al., Reference Mehlert, Zitzmann, Richardson, Treumann and Ferguson1998b). Recently, it has been reported that VSGs can also be O-glycosylated (Pinger et al., Reference Pinger, Nesic, Ali, Aresta-Branco, Lilic, Chowdhury, Kim, Verdi, Raper, Ferguson, Papavasiliou and Stebbins2018).
TfR is a heterodimeric protein expressed in the bloodstream form that is encoded by expression site-associated genes (ESAGs) 6 and 7 exhibiting eight N-glycosylation sequons. Both ESAG6 and ESAG7 are heterogeneously N-glycosylated with paucimannose and oligomannose moieties such as VSGs but not with complex N-glycans (Mehlert et al., Reference Mehlert, Wormald and Ferguson2012). In contrast to the glycoproteins described above, the N-glycosylation profile of the ISGs has not been characterized so far.
On the other hand, the GPI structure built-up by NH2CH2CH2-PO4H-6-Manα(1,2)-Manα(1,6)-Manα(1,4)-GlcNα(1,6)-myo-inositol-1-PO4H-3(sn-1,2-dimyristoylglycerol) by which the aforementioned glycoproteins, except for ISGs, are anchored to the surface membrane is further modified by N-glycosylation (Holder, Reference Holder1985; Ferguson et al., Reference Ferguson, Homans, Dwek and Rademacher1988; Redman et al., Reference Redman, Green, Thomas-Oates, Reinhold and Ferguson1994).
Glycans and interaction of T. brucei with the mammalian host
Trypanosoma brucei parasites dwelling in the mammalian bloodstream are exposed to innate and adaptive responses by the immune system for which they have developed sophisticated evasion strategies. An essential mechanism for effective immune evasion is the antigenic variation of VSGs whereby parasites switch to a new, immunologically distinct VSG, selected from among a huge collection of silent VSG genes. At the initial stages of the humoral immune response, when antibody levels are still low, the VSG–antibody complexes are rapidly internalized at the flagellar pocket by clathrin-dependent endocytosis, to be further dissociated in isolated VSG which is recycled to the surface, and the Ig that is directed to the lysosome to be proteolysed (O'Beirne et al., Reference O'Beirne, Lowry and Voorheis1998; Pal et al., Reference Pal, Hall, Jeffries and Field2003; Engstler et al., Reference Engstler, Thilo, Weise, Grunfelder, Schwarz, Boshart and Overath2004; Overath and Engstler, Reference Overath and Engstler2004). Antibody internalization becomes insufficient as the titre increases, and the complement system, mediated by specific antibodies against the predominant form of VSG, promotes efficient opsonization and lysis of parasites except for those expressing the new VSG that will spread again the infection, as they are able to escape the adaptive immune response. As the infection progresses, slender proliferative bloodstream parasites differentiate into a stumpy non-proliferative form that plays an important role in different ways. It contributes to avoid massive parasitaemia and premature host death, allows for pre-adaptation to the tsetse fly and by reducing the VSG repertoire expression it restricts antibody generation by the host, thus extending the functionality of antigenic variation (MacGregor et al., Reference MacGregor, Savill, Hall and Matthews2011; Matthews, Reference Matthews2015).
While trypanosomes mainly rely on antigenic variation to circumvent immune detection, VSG glycosylation modulates host–parasite interactions, contributing to the formation of an efficient surface barrier, with increased antigenic variability and protective properties (Blum et al., Reference Blum, Down, Gurnett, Carrington, Turner and Wiley1993). Supporting this notion, it has been shown that O-glycosylation of VSGs confers the parasite additional surface heterogeneity, impairs the functionality of the host immune response and enhances parasite virulence (Pinger et al., Reference Pinger, Nesic, Ali, Aresta-Branco, Lilic, Chowdhury, Kim, Verdi, Raper, Ferguson, Papavasiliou and Stebbins2018). Furthermore, specific carbohydrate branches at the trypanosome surface are involved in the process of binding and uptake of host macromolecules. The conserved VSGs chitobiose-oligomannose (GlcNAc2-Man5–9) moieties have been proposed to act as ligands for TNF-α, a cytokine with lectin-like properties inducing a pro-inflammatory response and parasite lysis (Fig. 2) (Magez et al., Reference Magez, Radwanska, Stijlemans, Xong, Pays and De Baetselier2001) although the direct induction of trypanolysis by TNF-α has been recently questioned (Vanwalleghem et al., Reference Vanwalleghem, Morias, Beschin, Szymkowski and Pays2017).
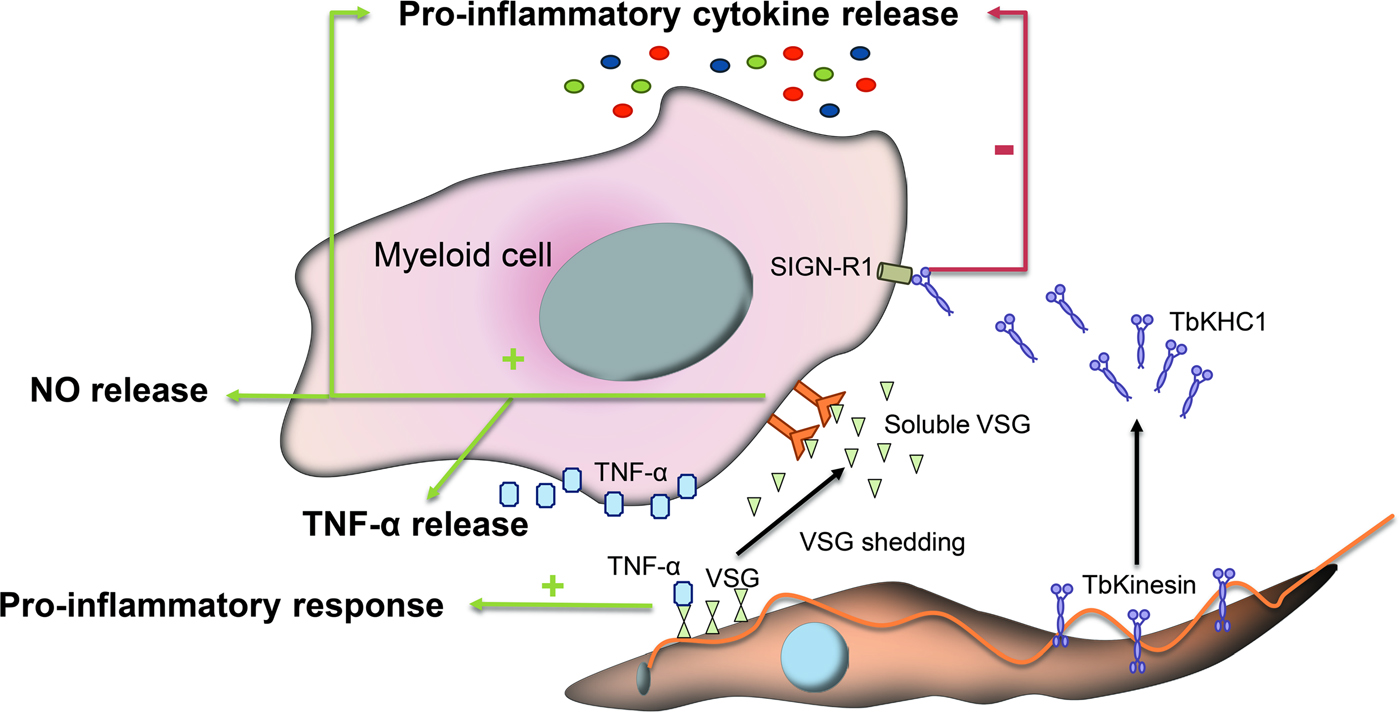
Fig. 2. Immunomodulatory events mediated by glycans during infection with T. brucei. VSGs interact with host immune cells and act as immunomodulatory factors. The conserved VSGs chitobiose-oligomannose moiety of VSGs binds to TNF-α, a cytokine with lectin-like properties and induces a pro-inflammatory response. Likewise, during differentiation to stumpy forms a VSG shedding process takes place allowing for the release of soluble VSG portions into the bloodstream of the mammalian host. These fragmented VSGs containing glycosylinositolphosphate induce myeloid cell activation and thereby the expression of pro-inflammatory cytokines and the release of NO and TNF-α. Other parasite-released factors interfere with the pro-inflammatory response such as the Kinesin Heavy Chain 1 (TbKHC1), which binds to the mannose-specific Intercellular Adhesion Molecule-3-Grabbing Nonintegrin-Related 1 (SIGN-R1) receptor and inhibits the host pro-inflammatory response.
VSGs also act as immunomodulatory factors involved in the production of TNF-α and nitric oxide (NO). During the differentiation process, slender trypanosomes suffer a VSG shedding process releasing to the bloodstream of the mammalian host soluble VSG portions from the membrane (Gruszynski et al., Reference Gruszynski, DeMaster, Hooper and Bangs2003). These fragmented VSGs containing glycosylinositolphosphate induce myeloid cell activation and thereby the expression of pro-inflammatory cytokines (Fig. 2) (Leppert et al., Reference Leppert, Mansfield and Paulnock2007). This process is amplified by T cell activation and IFN-γ release, which promotes macrophages to achieve a whole activated/M1 polarization and consequently increases TNF-α and NO secretion to control the infection (Stijlemans et al., Reference Stijlemans, Caljon, Van Den Abbeele, Van Ginderachter, Magez and De Trez2016).
Other parasite-released factors interfere with the pro-inflammatory response of activated macrophages thus contributing to parasite infection. For instance, Kinesin Heavy Chain 1 (TbKHC1) is released in the blood by the parasite and interacts with the mannose-specific intercellular adhesion molecule-3-grabbing nonintegrin-related 1 (SIGN-R1) receptor, a C-type lectin expressed in the surface of immune cells to inhibit the host pro-inflammatory response and at the same time stimulates the production by the host of essential trypanosomal nutrients (Fig. 2). TbKHC1 reduces the conversion of L-arginine into NO by inducing arginase-1 activity via IL-10 of macrophages/myeloid cells. The modulation of arginase activity promotes the formation of L-ornithine and consequently of polyamines required for trypanosome growth (De Muylder et al., Reference De Muylder, Daulouede, Lecordier, Uzureau, Morias, Van Den Abbeele, Caljon, Herin, Holzmuller, Semballa, Courtois, Vanhamme, Stijlemans, De Baetselier, Barrett, Barlow, McKenzie, Barron, Wynn, Beschin, Vincendeau and Pays2013).
Other glycoproteins that play major roles in host–parasite interaction are the trypanosome-derived lymphocyte-triggering factor, which is secreted by T. brucei parasites promoting early IFN-γ production by CD8+ T lymphocytes (Vaidya et al., Reference Vaidya, Bakhiet, Hill, Olsson, Kristensson and Donelson1997), the GPI-phospholipase C, a bloodstream stage-specific enzyme that is concentrated in the flagellar membrane and participates in VSG shedding during differentiation of bloodstream forms to procyclic forms (Grandgenett et al., Reference Grandgenett, Otsu, Wilson, Wilson and Donelson2007) and the TfR, which is located in the flagellar pocket and is involved in providing iron to the parasite (Steverding et al., Reference Steverding, Stierhof, Chaudhri, Ligtenberg, Schell, Beck-Sickinger and Overath1994). The N-glycosylation of both TfR protein subunits has been proposed to provide a spatial localization in the plasma membrane together with the VSG coat that allows transferrin binding without significant exposure to the immune system (Mehlert et al., Reference Mehlert, Wormald and Ferguson2012).
Surface glycans in Leishmania
In the case of Leishmania, the surface coat is covered by a dense external glycocalyx harbouring different glycoconjugates with an important role in the parasite–host interaction. Its nature varies between species and different forms of the parasite during the life cycle. Promastigote cells contain a series of glycoconjugates, which include GPI-anchored proteins such as the metalloprotease leishmanolysin/GP63, the parasite surface antigen-2 complex (PSA-2/GP46) and the mucin-like proteophosphoglycan (PPG), a complex GPI-anchored lipophosphoglycan (LPG) and low molecular weight GIPLs which are not attached to either proteins or polysaccharides. Leishmania also secretes protein-linked phosphoglycans, such as the secreted proteophosphoglycan (sPPG) and secreted acid phosphatase (Sacks and Kamhawi, Reference Sacks and Kamhawi2001).
LPG is the main cell-surface glycoconjugate in promastigotes covering the whole parasite including the flagellum. It is comprised a 1-O-alkyl-2-lyso-phosphatidyl(myo)inositol lipid anchor with a heptasaccharide glycan core, Galpβ(1,6)- Galpβ(1,3)-Galfα(1,3)(Glcβ(1)-PO4-(6))-Manβ(1,3)-Manβ(1,4)-GlcN, which is joined to a long polyglycosyl phosphate (PG) consisting of repeating units of a disaccharide and a phosphate (Galβ(1,4)-Manα(1)-PO4) and terminated by an oligosaccharide cap structure consisting of Manα(1,2)-Manα(1) or as Galβ(1,4)(Manα(1,2))-Manα(1). The PG units appear to be modified by carbohydrate chains that differ markedly between species and stage (McConville et al., Reference McConville, Schnur, Jaffe and Schneider1995). In amastigotes, LPG expression is highly downregulated (Turco and Sacks, Reference Turco and Sacks1991).
PPG is the second major phosphoglycan but, unlike LPG, it contains a polypeptide backbone rich in serines to which phosphoglycans are linked via phosphodiester bonds. The PG molecules consist of three differently branched phosphodisaccharides that end with a neutral capping structure (Ilg, Reference Ilg2000).
GP63 is the most abundant surface glycoprotein expressed on the Leishmania promastigote cell membrane. GP63 is N-glycosylated with paucimannose structures such as Man6GlcNAc2 and GlcMan6GlcNAc2 (Funk et al., Reference Funk, Thomas-Oates, Kielland, Bates and Olafson1997), and Man3GlcN structure in the GPI-anchor (Cabezas et al., Reference Cabezas, Legentil, Robert-Gangneux, Daligault, Belaz, Nugier-Chauvin, Tranchimand, Tellier, Gangneux and Ferrieres2015). It is a zinc-dependent protease with a wide range of substrates including casein, haemoglobin, fibrinogen, etc. (Yao et al., Reference Yao, Donelson and Wilson2003).
Differentiation to the amastigote form involves the thinning of the glycocalyx; in addition to LPG, the levels of GP63 are also dramatically decreased and GIPLs become the major surface glycoconjugate in this form (McConville and Blackwell, Reference McConville and Blackwell1991; Schneider et al., Reference Schneider, Rosat, Bouvier, Louis and Bordier1992; Winter et al., Reference Winter, Fuchs, McConville, Stierhof and Overath1994). GIPLs are composed of the Manα(1,4)-GlcNα(1,6)-myo-inositol unit, which is substituted with either high mannose (type-1) or Galp-Galf (type-2) structures or with both forming a hybrid glycoside (McConville and Ferguson, Reference McConville and Ferguson1993; Cabezas et al., Reference Cabezas, Legentil, Robert-Gangneux, Daligault, Belaz, Nugier-Chauvin, Tranchimand, Tellier, Gangneux and Ferrieres2015). Like in T. cruzi, Leishmania also presents its cell surface decorated with sialic acid-bearing glycoconjugates. Leishmania donovani promastigotes exhibit 9-O-acetylated sialic acid and distinct 9-O-acetylated sialoglycoproteins while the amastigote form harbours an unusual derivative of sialic acid, Neu5Gc, absent in promastigotes (Ghoshal and Mandal, Reference Ghoshal and Mandal2011).
Role of Leishmania surface glycans in immunomodulation and parasite–host cell interactions
The presence of a cell surface glycocalyx has a critical role in host–parasite interactions and infectivity thanks to an array of well-defined epitopes of branched N-glycans that act as ligands for receptors on cells of the insect or the vertebrate host. In Leishmania promastigotes, the dense glycocalyx formed by LPG performs a number of functions for parasite survival within the insect and for macrophage infection within the mammalian host. LPGs confer physical protection against digestive hydrolytic enzymes of the sandfly and are involved in the attachment to the gut epithelium and migration of metacyclic parasites to the mouthparts of the insect (Ilg, Reference Ilg2000; Sacks and Kamhawi, Reference Sacks and Kamhawi2001). In the blood stream, LPG prevents lysis by complement proteins and serves as a ligand for attachment and receptor-mediated phagocytosis by the macrophage. LPG triggers TLR signalling and interferes with pro-inflammatory and signalling pathways in host cells (Fig. 3) (Becker et al., Reference Becker, Salaiza, Aguirre, Delgado, Carrillo-Carrasco, Kobeh, Ruiz, Cervantes, Torres, Cabrera, Gonzalez, Maldonado and Isibasi2003; Rojas-Bernabe et al., Reference Rojas-Bernabe, Garcia-Hernandez, Maldonado-Bernal, Delegado-Dominguez, Ortega, Gutierrez-Kobeh, Becker and Aguirre-Garcia2014). Once inside the macrophage, LPG delays the fusion of the parasitophorous vacuole with lysosomes and inhibits protein kinase C and the production of cytokines related to the microbicidal oxidative and nitrosative stress response (Fig. 3) (Descoteaux and Turco, Reference Descoteaux and Turco1993; Kavoosi et al., Reference Kavoosi, Ardestani and Kariminia2009; Franco et al., Reference Franco, Beverley and Zamboni2012).

Fig. 3. Schematic representation of the major parasite–macrophage interactions mediated by surface glycans in Leishmania. The major glycoconjugates involved in the parasite–macrophage interplay are indicated: PPG, GPI-anchored LPG GIPLs and the metalloprotease GP63. After infection, promastigote LPG triggers TLR signalling and interferes with pro-inflammatory and signalling pathways. Once inside the macrophage, LPG delays the fusion of the parasitophorous vacuole with lysosomes and inhibits protein kinase C and therefore, the production of cytokines and the oxidative and nitrosative stress response. Likewise, mannose-terminating GIPLs interact with mannose receptors on the macrophage surface and inhibit PKC activity. sPPGs impair important macrophage functions such as the release of TNF-α. Finally, GP63 is an important virulence factor which, among other functions, promotes Leishmania internalization and facilitates escape from lysis by the complement pathway.
Besides LPG, other glycoconjugates such as GIPLs and PPGs are involved in the first stages of macrophage infection (McConville and Blackwell, Reference McConville and Blackwell1991; Piani et al., Reference Piani, Ilg, Elefanty, Curtis and Handman1999). Mannose-terminating GIPLs interact with mannose receptors on the macrophage surface (Blackwell et al., Reference Blackwell, Ezekowitz, Roberts, Channon, Sim and Gordon1985) and modulate many macrophage functions such as PKC activity (Chawla and Vishwakarma, Reference Chawla and Vishwakarma2003), cytokine production, release of NO and differentially activate MAPK (Fig. 3) (Assis et al., Reference Assis, Ibraim, Noronha, Turco and Soares2012). While the implication of GIPLs in Leishmania–macrophages interaction is well established, their role in intramacrophage development is still unclear. On the other hand, PPGs play important biological roles in the establishment of Leishmania infection and virulence (Capul et al., Reference Capul, Hickerson, Barron, Turco and Beverley2007; Gaur et al., Reference Gaur, Showalter, Hickerson, Dalvi, Turco, Wilson and Beverley2009; Olivier et al., Reference Olivier, Atayde, Isnard, Hassani and Shio2012). Filamentous PPGs are found in the promastigote secretory gel, a viscous mucin-like material which accumulates in sandfly gut and mouthparts and improves Leishmania transmission by promoting multiple insect bites and increasing the number of parasites per bite (Rogers et al., Reference Rogers, Ilg, Nikolaev, Ferguson and Bates2004; Rogers and Bates, Reference Rogers and Bates2007). PPGs regurgitated by Leishmania-infected sandflies favour macrophage recruitment to the bite site and target the L-arginine metabolism of host macrophages to promote establishment of the infection (Rogers et al., Reference Rogers, Kropf, Choi, Dillon, Podinovskaia, Bates and Muller2009). In a murine model, sPPG has been shown to inhibit TNF-α release to facilitate the establishment of the infection (Piani et al., Reference Piani, Ilg, Elefanty, Curtis and Handman1999).
GPI-anchored glycoproteins that exhibit pivotal functions in the parasite–mammalian host interplay in Leishmania are GP63 and PSA-2. Metalloprotease GP63 is abundant in promastigotes but expressed to a lesser extent in amastigotes (Schneider et al., Reference Schneider, Rosat, Bouvier, Louis and Bordier1992). GP63 is an important virulence factor which modulates a wide range of host cell signalling pathways that regulate macrophage anti-microbial and inflammatory functions. GP63 facilitates parasite escape from lysis by the complement pathway and the movement through the extracellular matrix, favours promastigote internalization into macrophages, inhibits natural killer cell responses, promotes resistance to antimicrobial peptides and seems to play a key role in protecting intracellular parasites from the hostile environment of macrophages (Fig. 3) (Olivier et al., Reference Olivier, Atayde, Isnard, Hassani and Shio2012; Yao et al., Reference Yao, Donelson and Wilson2003, Reference Yao, Li, Donelson and Wilson2010). Likewise, the PSA-2 protein is involved in the binding and invasion of parasites on macrophages and resistance to complement lysis (Kedzierski et al., Reference Kedzierski, Montgomery, Bullen, Curtis, Gardiner, Jimenez-Ruiz and Handman2004; Lincoln et al., Reference Lincoln, Ozaki, Donelson and Beetham2004).
Another type of interaction with host cells by which parasites can establish successful infection takes place through sialic acid-siglec binding. Leishmania utilizes sialic acids to bind these membrane-bound receptors present in the haematopoetic cell lineages promoting parasite entry within macrophages, NO-resistance, host immunomodulation and strain virulence (Fig. 3) (Ghoshal and Mandal, Reference Ghoshal and Mandal2011; Roy and Mandal, Reference Roy and Mandal2016).
Carbohydrate-binding agents as antimicrobials
Several studies support the therapeutic potential of carbohydrate-binding agents (CBAs). Lectins are CBAs of peptidic nature that specifically bind diverse carbohydrate structures. By acting as recognition and adhesion molecules and as signal transducers they perform a wide variety of physiological functions. Cell membrane proteins and lipids in many pathogens exhibit specific glycosylation patterns different from the mammalian host and are potential binding sites for lectins of selected specificity. Thus, lectins naturally occurring in plants, microbes, animals and humans exhibit antimicrobial activity (Petrova et al., Reference Petrova, Imholz, Verhoeven, Balzarini, Van Damme, Schols, Vanderleyden and Lebeer2016; Zhang and Gallo, Reference Zhang and Gallo2016) through the interaction with complex carbohydrates on microbial surfaces and there is growing interest in their applicability due to the possible interference with host cell–pathogen interactions and disease development (Breitenbach Barroso Coelho et al., Reference Breitenbach Barroso Coelho, Marcelino Dos Santos Silva, Felix de Oliveira, de Moura, Viana Pontual, Soares Gomes, Guedes Paiva, Napoleao and Dos Santos Correia2018). Decreased capacity of invasion and infection, inhibition of proliferation and impairment of pathogen cell adhesion and migration has been reported to occur upon incubation with lectins from different sources (da Silva et al., Reference da Silva, da Silva, da Silva, Vieira, de Araujo, de Albuquerque Lima, de Oliveira, do Nascimento Carvalho, da Rocha Pitta, de Melo Rego, Pinheiro, Zingali, do Socorro de Mendonca Cavalcanti, Napoleao and Paiva2019; Hasan and Ozeki, Reference Hasan and Ozeki2019; Li et al., Reference Li, Yu, Zhang, Cheng, Hou, Zheng and Hou2019). In addition, lectins have been acknowledged as promising potential carrier molecules for directed drug delivery (Žurga et al., Reference Žurga, Nanut, Kos and Sabotic2017) since by binding to membrane glycan moieties, they can elicit cell internalization of molecules of therapeutic interest.
Indeed with regards to their anti-infective potential, multiple studies have demonstrated the enormous antiviral capacities of CBAs (Francois and Balzarini, Reference Francois and Balzarini2012; Gondim et al., Reference Gondim, Roberta da Silva, Mathys, Noppen, Liekens, Holanda Sampaio, Nagano, Renata Costa Rocha, Nascimento, Cavada, Sadler and Balzarini2019). The infectivity of several viruses requires surface glycoproteins and interference with host cell recognition has been the basis of the antiviral activity of lectins that specifically bind mannose-rich surface glycans (Dey et al., Reference Dey, Lerner, Lusso, Boyd, Elder and Berger2000; Hoorelbeke et al., Reference Hoorelbeke, Huskens, Ferir, Francois, Takahashi, Van Laethem, Schols, Tanaka and Balzarini2010). Prolonged exposure to CBAs resulted in defects in the glycosylation status of surface glycoproteins giving rise to defective binding and increased exposure of underlying epitopes to the host immune response adding this way a new feature to their mode of action (Balzarini et al., Reference Balzarini, Van Laethem, Hatse, Froeyen, Peumans, Van Damme and Schols2005). In addition, non-peptidic CBAs have been successfully used in the treatment of fungal infections both in vitro and in vivo supporting the possible employment of this class of compounds in a clinical setting (Tomita et al., Reference Tomita, Nishio, Saitoh, Yamamoto, Hoshino, Ohkuma, Konishi, Miyaki and Oki1990; Walsh and Giri, Reference Walsh and Giri1997).
Therapeutic opportunities in kinetoplastids
In the case of kinetoplastid diseases, treatment often suffers from toxicity, side-effects and limited efficacy. New entities with novel modes of action are therefore needed to address the increasing demand for novel medicines. Despite extensive screening and in vitro and in vivo studies, only very few compounds have advanced to clinical trials. Taking into account the importance of protein glycosylation, the unique character of cell surface glycans during the infective stages of parasitic protozoa opens exciting possibilities for the use of CBAs as antiparasitics. A mode of action can be foreseen where these agents would act directly exerting toxicity by binding to the cell surface and inducing parasite lysis and/or additionally by preventing pathogen infection in the host by impairing crucial interactions involved in the attenuation of the immune response or parasite uptake. The direct cytotoxic activity upon incubation with CBAs has been demonstrated in T. brucei bloodstream forms where peptidic agents such as the amaryllis lectin Hippeastrum hybrid (HHA) and the stinging nettle lectin (UDA) from Urtica dioica perturb endocytosis (Castillo-Acosta et al., Reference Castillo-Acosta, Vidal, Ruiz-Perez, Van Damme, Igarashi, Balzarini and Gonzalez-Pacanowska2013, Reference Castillo-Acosta, Ruiz-Perez, Van Damme, Balzarini and Gonzalez-Pacanowska2015). Likewise, a specific block in endocytosis was observed after exposure of T. cruzi to a Poly-LAcNAc binding lectin (Brosson et al., Reference Brosson, Fontaine, Vermeersch, Perez-Morga, Pays, Bousbata and Salmon2016). However, while many plant peptidic CBAs exist with a wide range of glycan specificities, including mannose, galactose, glucose, fucose, sialic acid, GlcNAc and GalNAc oligomers, the approach involves major challenges. The toxicity of many peptidic lectins precludes their use as drugs and the identification of CBAs that exhibit selectivity towards parasite glycans and low toxicity towards mammalian cells are required.
Previous studies on the utility of CBAs in kinetoplastid diseases are limited. Certain plant lectins have demonstrated utility as adjuvants when studying the mouse humoral immune response to T. cruzi (Albuquerque et al., Reference Albuquerque, Martins, Campos-Neto and Silva1999). The cramoll 1,4 lectin, is a protein that recognizes and interacts with specific glycans on the cell surface inducing mitogenic activity (Maciel et al., Reference Maciel, Araujo-Filho, Nakazawa, Gomes, Coelho and Correia2004) which in T. cruzi, induces changes in plasma membrane permeability, production of reactive oxygen species and defects in mitochondrial function (Fernandes et al., Reference Fernandes, Inada, Chiaratti, Araujo, Meirelles, Correia, Coelho, Alves, Gadelha and Vercesi2010). A protective effect of lectin administration in Leishmania infections has also been documented. Thus, lectins such as the ConBr from Canavalia brasiliensis and KM+ from Artocarpus integrifolia induce IFN-γ and IL-12 p40 production promoting a reversal of the Th2 cytokine pattern to Th1 pattern in BALB/c mice infected with Leishmania amazonensis and Leishmania major, respectively (Barral-Netto et al., Reference Barral-Netto, Von Sohsten, Teixeira, dos Santos, Pompeu, Moreira, Oliveira, Cavada, Falcoff and Barral1996; Panunto-Castelo et al., Reference Panunto-Castelo, Souza, Roque-Barreira and Silva2001). Pretreatment of murine inflammatory peritoneal macrophages with a D-galactose-binding lectin from Synadenium carinatum latex (ScLL) reduced by 65.5% the association index of macrophages and L. amazonensis promastigotes (Afonso-Cardoso et al., Reference Afonso-Cardoso, Silva, Ferreira and Souza2011). ScLL also reduced the growth of L. amazonensis amastigote intracellular forms, showing no in vitro cytotoxic effects in mammalian host cells (Afonso-Cardoso et al., Reference Afonso-Cardoso, Silva, Ferreira and Souza2011).
Remarkably, evidence has been recently presented showing that the use of CBAs could involve a completely novel approach to chemotherapy of protozoan-infectious diseases in the case of sleeping sickness. Thus products from natural sources of both peptidic and non-peptidic nature have demonstrated their antitrypanosomal potential in the case of T. brucei both in vitro and in vivo (Castillo-Acosta et al., Reference Castillo-Acosta, Vidal, Ruiz-Perez, Van Damme, Igarashi, Balzarini and Gonzalez-Pacanowska2013, Reference Castillo-Acosta, Ruiz-Perez, Etxebarria, Reichardt, Navarro, Igarashi, Liekens, Balzarini and Gonzalez-Pacanowska2016). Certain α(1,3)-α(1,6)Man-specific peptidic CBAs such as the HHA or the α(1,3)Man-specific snowdrop lectin from Galanthus nivalis (GNA) inhibit growth of bloodstream forms of T. brucei while exhibiting very low toxicity against mammalian cells in vitro (Castillo-Acosta et al., Reference Castillo-Acosta, Vidal, Ruiz-Perez, Van Damme, Igarashi, Balzarini and Gonzalez-Pacanowska2013) although as peptides their therapeutic potential was limited. In the case of non-peptidic glycan binding agents, the identification of low molecular weight, non-toxic compounds to date has been restricted mostly to the natural products from Actinomycetes namely pradimicin A (Walsh and Giri, Reference Walsh and Giri1997) and benanomicin A (Watanabe et al., Reference Watanabe, Hiratani, Uchida, Ohtsuka, Watabe, Inouye, Kondo, Takeuchi and Yamaguchi1996) and their synthetic analogues although several groups have aimed at the synthesis and characterization of synthetic CBAs that bind specific oligosaccharide structures (Striegler and Dittel, Reference Striegler and Dittel2003; Mazik et al., Reference Mazik, Cavga and Jones2005). Indeed treatment with the non-peptidic CBA pradimicin S procured parasitological cure in mouse models of acute sleeping sickness with no evidence of toxicity or side-effects further supporting the potential of the approach (Castillo-Acosta et al., Reference Castillo-Acosta, Ruiz-Perez, Etxebarria, Reichardt, Navarro, Igarashi, Liekens, Balzarini and Gonzalez-Pacanowska2016). Pradimicin A exhibits α(1,2)Man specificity and binds tightly to the parasite VSGs presumably through specific interactions with oligomannose surface glycans that are highly abundant in the bloodstream form of the parasite. Binding of pradimicin to bloodstream form trypanosomes induced defects in endocytosis and parasite lysis. Interestingly induction of resistance to pradimicin A in vitro resulted in parasites with defective glycosylation and a reduction in the content of mannose-rich glycans that exhibited reduced infectivity thus clearly supporting the proposed mechanism of action (Castillo-Acosta et al., Reference Castillo-Acosta, Ruiz-Perez, Etxebarria, Reichardt, Navarro, Igarashi, Liekens, Balzarini and Gonzalez-Pacanowska2016). Binding to mannose-rich surface glycoproteins was also the basis for the potent activity of pradimicins against HIV by the interaction with the heavily mannosylated surface glycoprotein gp120 (Balzarini et al., Reference Balzarini, Van Laethem, Hatse, Froeyen, Peumans, Van Damme and Schols2005).
When understanding the antiprotozoal activity of CBAs, as previously mentioned, the complement system, mediated by specific antibodies against VSGs, allows for efficient opsonization and lysis of parasites. Considering the capability of CBAs to interact with T. brucei membrane glycoproteins and in particular VSGs, we hypothesize that CBAs could act as opsonins per se and therefore increase phagocytosis by macrophages. In addition, it is possible that the endocytosis block mediated by CBAs may also interfere with VSG recycling leading to a reduced clearance of surface coat antibodies and further promoting the opsonization process. On the other hand, CBAs may bind to specific carbohydrate branches at the trypanosome surface that are crucially involved in the process of binding of host macromolecules. Thus in T. brucei transferrin binding by the TfR could also be compromised (Mehlert et al., Reference Mehlert, Wormald and Ferguson2012). Furthermore, TbKHC1 interacts with the mannose-specific SIGN-R1 receptor and inhibits the pro-inflammatory response of the host (De Muylder et al., Reference De Muylder, Daulouede, Lecordier, Uzureau, Morias, Van Den Abbeele, Caljon, Herin, Holzmuller, Semballa, Courtois, Vanhamme, Stijlemans, De Baetselier, Barrett, Barlow, McKenzie, Barron, Wynn, Beschin, Vincendeau and Pays2013). Again it is possible that CBAs interfere with these or other events that may be related to its mode of action in vivo.
In the case of T. cruzi, the core of GIPLs, a major surface constituent, is made up by Manα(1,2)-Manα(1,6)-Manα(1,4)-GlcNα(1,6)-myo-inositol-PO4-lipid (McConville and Ferguson, Reference McConville and Ferguson1993) while galactose can be found attached to different positions. As previously mentioned TcCRT, which interacts with host PRRs mannose-binding lectins, increases host cell parasite internalization. Perturbation of this process through the use of mannose binding CBAs could reduce infection of new cells. The abundant mucins contain galactose in their oligosaccharide elongations and are O-glycosylated with GlcNAc. In addition, the exposure of free β(1,3)Gal residues and β-Gal residues has been suggested to mediate parasite attachment and entry in dendritic cells. Indeed, human galectin-3, a member of the lectin family with affinity to β-Gal and derivatives, plays a pivotal role in controlling T. cruzi infection. It has been recently proposed that galectin-3 deficiency during T. cruzi experimental infection resulted in increased in vivo systemic parasitaemia, and reduced leucocyte recruitment (da Silva et al., Reference da Silva, Teixeira, Teixeira, Machado, Dos Santos, Tomiosso, Tavares, Brigido, Martins, Silva, Rodrigues, Roque-Barreira, Mortara, Lopes, Avila and da Silva2017). Since galactose-specific lectins are available, their possible interaction with infection mechanisms in the case of T. cruzi warrants investigation of the potential of this class of CBAs in the case of Chagas disease.
Finally, the use of treatment with CBAs in the case of infections caused by Leishmania spp. should also be further considered. Leishmania can target several macrophage membrane-bound receptors to subvert the inflammatory response. Mannose-terminating GIPLs interact with mannose receptors on the macrophage surface (Blackwell et al., Reference Blackwell, Ezekowitz, Roberts, Channon, Sim and Gordon1985) modulating macrophage functions such as PKC activity (Chawla and Vishwakarma, Reference Chawla and Vishwakarma2003), cytokine levels and the production of NO. Sialic acids on the parasite surface interact with siglec receptors on macrophages to diminish the immune response (Roy and Mandal, Reference Roy and Mandal2016). Additionally, of interest is the observation that TLR-2 is involved in parasite survival in macrophages upon activation by LPG and interactions between LPG and TLR-2 reduce anti-leishmanial responses via cytokine-mediated decrease of TLR-9 expression (Srivastava et al., Reference Srivastava, Pandey, Jha, Chandel and Saha2013). All these important mechanisms of parasite survival may be perturbed upon treatment with mannose-specific CBAs.
Concluding remarks
As highlighted in the present review, kinetoplastids interact with mammalian host cells by recognizing specific glycan ligands. Parasite surface carbohydrates are involved in parasite attachment and entry as well as in the modulation of the immune response and the progression of infection. Based on observations with lectins and non-peptidic pradimicins in the case of sleeping sickness, the use of CBAs emerges as a promising antitrypanosomal strategy. Specificity of the interactions and the unique structure of kinetoplastid surface sugars may provide a basis for future drug design. Many bioactive natural carbohydrate-binding compounds are present in nature that often exhibit exquisite specificity for binding to carbohydrates, particularly carbohydrate sequences that occur on the surface of living cells. These molecules have the potential for treatment of kinetoplastid diseases. While specificity and toxicity may constitute important issues, the possibility of identifying agents that can be used to block the attachment of the parasite to cell surfaces (or interfere with the subversion of the immune response), and thus prevent or suppress infection is appealing. Future identification of new CBAs with improved pharmacological profiles and reduced side-effects may provide novel avenues for the exploitation of this innovative concept.
Acknowledgements
The authors thank Dr Santiago Castanys Cuello for critical reading of the manuscript.
Financial support
Financial support was received from the Junta de Andalucía (BIO-199 P12-BIO-2059); the Plan Nacional, Instituto de Salud Carlos III-Subdirección General de Redes y Centros de Investigación Cooperativa-Red de Investigación Cooperativa en Enfermedades Tropicales (RICET: RD16/0027/0014) and the Plan Nacional de Investigación Científica (SAF2016-79957-R) and the FEDER funds from the EU.
Conflict of interest
None.
Ethical standards
Not applicable.






