Introduction
Single-species parasite infections have received significant attention (e.g. Lefèvre et al., Reference Lefèvre, Lebarbenchon, Gauthier-Clerc, Missé, Poulin and Thomas2008; Lafferty, Reference Lafferty2017), but multi-parasite infections are increasingly understood as significant drivers of host health and disease outbreaks (Hellard et al., Reference Hellard, Fouchet, Vavre and Pontier2015; Clay and Rudolf, Reference Clay and Rudolf2019). Impacts of parasite infections may include decreased host immune function, increased parasite fitness (Pedersen and Fenton, Reference Pedersen and Fenton2007; Budischak et al., Reference Budischak, Sakamoto, Megow, Cummings, Urban and Ezenwa2015), induction of severe pathologies (Gleichsner et al., Reference Gleichsner, Reinhart and Minchella2018) and altered host response to future infections (Rodríguez et al., Reference Rodríguez, Terrazas, Márquez and Bojalil1999; Graham, Reference Graham2008). Co-infections by multiple parasites can either relieve or exacerbate these impacts on host health at both the individual and population level (Reckardt and Kerth, Reference Reckardt and Kerth2009; Hellard et al., Reference Hellard, Fouchet, Vavre and Pontier2015), such that a community-level understanding of parasite infections is important for quantifying parasite impacts on host health.
Commercially important finfish and crustaceans commonly serve as hosts for a diversity of parasites in freshwater and marine environments and wherever possible their presence and impacts should be incorporated into management approaches (Marcogliese, Reference Marcogliese2004; Byers, Reference Byers2021). For example, over the course of their life cycle, white shrimp, Penaeus setiferus (L., 1767) (Decapoda: Penaeidae) serve as hosts to a variety of parasites, including but not limited to, platyhelminthes, nematodes, ciliates and microsporidians (e.g. Overstreet, Reference Overstreet1978; Domínguez-Machín et al., Reference Domínguez-Machín, Hernández-Vergara, Jiménez-García, Simá-Álvarez and Rodríguez-Canul2011; Del Río-Rodríguez et al., Reference Del Río-Rodríguez, Pech, Soto-Rodriguez, Gomez-Solano and Sosa-Lopez2013). Penaeid shrimp are one of the most important fisheries and aquaculture industries worldwide (FAO, 2018). Specifically, adult white shrimp support important commercial and recreational fisheries along the coasts of the southeastern USA (Gillet, Reference Gillet2008) and the Gulf of Mexico (Muncy, Reference Muncy1984; NMFS, 2020), including $13.7 million annually from the Georgia Bight in the western Atlantic (Kendrick et al., Reference Kendrick, Brunson, Frischer and Kingsley-Smith2021). Additionally, white shrimp provide a food source for many recreationally and ecologically important finfish, including red drum, Sciaenops ocellatus (see Scharf and Schlight, Reference Scharf and Schlight2000) and spotted seatrout, Cynoscion nebulosus (see Fujiwara et al., Reference Fujiwara, Zhou, Acres and Martinez-Andrade2016). As such, white shrimp comprise an integral part of the estuarine ecosystem and economy and monitoring population health is necessary to ensure sustainable management.
Commercial landings of white shrimp in parts of the Georgia Bight have declined during the last 2 decades (Kendrick et al., Reference Kendrick, Brunson, Frischer and Kingsley-Smith2021), coinciding with numerous biotic and abiotic changes in the estuarine waters of the southeastern USA (e.g. Hollebone, Reference Hollebone2006; Shearman and Lentz, Reference Shearman and Lentz2010). Among these biotic changes is the increased prevalence of a condition known as black gill (Fowler et al., Reference Fowler, Leffler, Johnson, DeLancey and Sanger2018; Swinford and Anderson, Reference Swinford and Anderson2021; Tuckey et al., Reference Tuckey, Swinford, Fabrizio, Small and Shields2021). This condition refers to the melanization of gill tissues that occurs as part of an immune response against irritants or pathogens (Vaseeharan and Ramasamy, Reference Vaseeharan and Ramasamy2003; Johnson et al., Reference Johnson, Burnett and Burnett2011; Burnett and Burnett, Reference Burnett and Burnett2015; Karthikeyan et al., Reference Karthikeyan, Selvakumar and Gopalakrishnan2015; Frischer et al., Reference Frischer, Landers, Walker, Powell and Lee2022). Black gill in wild-caught penaeid shrimp has been associated with the presence of the apostome ciliate Hyalophysa lynni (see Landers et al., Reference Landers, Lee, Walters, Walker, Powell, Patel and Frischer2020), but also occurs in response to heavy metals (Ghate, Reference Ghate1984), bacteria (Vaseeharan and Ramasamy, Reference Vaseeharan and Ramasamy2003) and fungi (Karthikeyan et al., Reference Karthikeyan, Selvakumar and Gopalakrishnan2015).
Parasites of white shrimp in the Georgia Bight, including South Carolina (SC) have not been intensively surveyed, and their impacts on white shrimp health at the individual and population level, and consequently on commercial landings, warrant investigation. The objectives of this study were to: (1) identify parasite community composition of white shrimp in the Charleston Harbor, SC; (2) document ontogenetic and spatial variability in the parasite communities of white shrimp; and (3) assess the relationships between parasites and black gill in white shrimp.
Material and methods
Collection of post-larval white shrimp
Zooplankton sampling was conducted at the surface during nocturnal flood tides that coincided with neap tides to correspond with the timing of shrimp ingress (DeLancey et al., Reference DeLancey, Jenkins and Whitaker1994; Wenner et al., Reference Wenner, Knott, Barans, Wilde, Blanton and Amft2005). Collections occurred between 6 June 2018 and 1 September 2018 and consisted of 18 sampling events. Two 0.75 m diameter plankton nets (500 μm mesh) were deployed for 30–75 min (depending on changes in tidal flow or the timing of the flood tide with respect to darkness) from a floating dock in Charleston Harbor, SC, USA (Fig. 1). Plankton samples were washed with seawater using a 500 μm mesh sieve and transported in an aerated container of seawater to the laboratory. Within 24 h, post-larval shrimp were isolated using a dissecting microscope and identified as penaeid following the key of Johnson and Allen (Reference Johnson and Allen2005). These specimens were presumed to be white shrimp based on the timing of ingress, which largely does not overlap with any other penaeid species in SC (DeLancey et al., Reference DeLancey, Jenkins and Whitaker1994). Specimens were flattened between 2 slides and examined whole under a compound microscope to detect parasites.

Fig. 1. Map of sampling localities in the greater Charleston Harbor, South Carolina, USA: Wando River, Ashley River and Charleston Harbor. Credit: Gary Sundin, South Carolina Department of Natural Resources (SCDNR).
Collection of juvenile, subadult and adult white shrimp
Juvenile, subadult and adult white shrimp were collected within the greater Charleston Harbor watershed at each of 3 localities (Fig. 1); tidal creek localities in the Ashley River watershed (n = 3), tidal creek localities in the Wando River watershed (n = 3) and open water localities in Charleston Harbor (n = 2). Specimens were collected from tidal creek localities monthly from June to September 2018 (n = 15 per site) and following their egress from tidal creeks in late-summer, from open water localities monthly (n = 23 per site) from August to November 2018. Collections from tidal creek localities were made using a 3.05 m otter trawl (0.95 cm stretch mesh net) towed for 5 min at ~2 knots. Collections from open water localities were made using a 6.2 m otter trawl (2.5 cm stretch mesh net) for 15 min at ~2 knots. Bottom water temperature (°C) and salinity (psu) were recorded at each site on each collection date using a handheld YSI Pro2030. Shrimp were measured from the tip of the rostrum to the tip of the telson, and size classes were defined according to Whitaker and Kingsley-Smith (Reference Whitaker and Kingsley-Smith2014) as follows: juvenile (⩽114 mm length), subadult (115–126 mm length) and adult (⩾127 mm length).
Identification of parasite community composition
Specimens were brought back on ice to the laboratory and examined for parasites immediately, or stored at –20°C for later processing. To collect parasites, individual shrimp were submerged in a dish of deionized water and, using a dissecting microscope, the carapace was removed and the organs excised from the cephalothoracic cavity. The hepatopancreas and feeding palps were deconstructed to isolate parasites, while the nerve cord was resected from the full length of the shrimp, flattened between 2 slides and examined with a compound microscope under 100× total magnification. The anterior caecum was resected from the digestive tract and opened along with the stomach, intestine and hindgut to examine their contents. A ~1–2 mm2 squash of the abdominal muscle was performed between 2 slides and examined at 100× total magnification. Smears were performed when gonads or muscle were infected and stained with Giemsa. Parasite specimens were fixed in 95% ethanol for molecular analysis.
As morphological identification of larval parasites can be challenging, morphotypes of parasite groups were first developed using morphological characteristics and infection sites (Overstreet, Reference Overstreet1973, Reference Overstreet1978; Deardorff and Overstreet, Reference Deardorff and Overstreet1981; Palm, Reference Palm2004; Sokolova et al., Reference Sokolova, Pelin, Hawke and Corradi2015). These morphotypes were screened using genetic sequencing for further identification. Subsamples of each morphotype group (except for the sessilid ciliates, which were not preserved) were processed for molecular analyses. DNA extractions (n = 26) were performed using a DNeasy Blood and Tissue kit (Qiagen, Valencia, CA, USA) following manufacturer protocols, except that elution volumes were reduced to 40 μL. For each taxonomic group, primers were chosen that would amplify a gene region most likely to provide species-level identifications (Table 1).
Table 1. Primers used for amplification and sequencing of white shrimp parasites

The gene region used was based on the most informative marker for species-level identification: large subunit ribosomal RNA (LSU rRNA) gene for platyhelminthes, partial small subunit (SSU) rRNA gene for ciliates and microsporidians, and mitochondrial cytochrome c oxidase II (cox2) for nematodes.
For primer orientation, +, sense; −, antisense.
For platyhelminthes, a 25 μL total reaction contained 1× Promega GoTaq® Flexi PCR Buffer (Madison, WI, USA), 0.1× Invitrogen Rediload™ loading buffer (Thermo Fisher Scientific, Waltham, MA, USA), 1.5 mm MgCl2, 0.2 mm dNTPs, each primer at 0.4 μ m, 0.05 U μL−1 Promega GoTaq® DNA polymerase and 1 μL template DNA. Cycling was performed as described by Jensen and Bullard (Reference Jensen and Bullard2010), with one alteration in that cycling began with a 5 min denaturation at 95 °C rather than at 94 °C. For ciliates, PCR reagents and concentrations were the same as above except 0.5 μ m of each primer was used. Cycling was performed as described by Guo et al. (Reference Guo, Liu, Hu, Li, Huang, Liu, Zhang and Lin2012). For nematodes, a 25 μL total reaction contained 1× PCR Buffer (Thermo Fisher Scientific), 0.1× Invitrogen Rediload™ loading buffer (Thermo Fisher Scientific), 2.5 mm MgCl2, 0.2 mm dNTPs, each primer at 0.2 μ m, 0.2 μ m Invitrogen Platinum™ Taq DNA polymerase and 1 μL template DNA. Cycling was performed as follows: 5 min at 96 °C then 40 cycles at 96 °C for 40 s, 45 °C for 40 s and 72 °C for 40 s, then 72 °C for 5 min. For microsporidians, PCR reagents and concentrations were the same as for the platyhelminthes PCR (described above), except that 1 μ m of each primer was used in a 20 μL total reaction volume. Cycling performed was as follows: 4 min at 94 °C then 35 cycles at 94 °C for 30 s, 55 °C for 30 s and 72 °C for 2 min and then at 72 °C for 5 min. For gregarines, several small subunit ribosomal RNA gene primers (Leander et al., Reference Leander, Clopton and Keeling2003; Rueckert and Leander, Reference Rueckart and Leander2009; Diakin et al., Reference Diakin, Paskerova, Simdyanov, Aleoshin and Valigurová2016) were tested but did not result in amplification.
All PCR products were electrophoresed on 1% agarose gels (100 V, 30 min) stained with GelRed® (Biotium, Inc., Hayward, CA, USA), and visualized under UV light. Products were cleaned using ExoSAP-IT™ (Affymetrix, Santa Clara, CA, USA) following manufacturer protocols. Cleaned products were sent to Eurofins MWG Operon LLC (Louisville, KY, USA) for direct, bi-directional sequencing using the primers shown in Table 1. Complementary sequences were compared to one another and to their chromatograms using Sequencher® version 5.4 (Gene Codes, Ann Arbor, MI, USA). Resulting sequences were compared against the NCBI GenBank database using the Basic Local Alignment Search Tool (BLAST; Altschul et al., Reference Altschul, Gish, Miller, Myers and Lipman1990). A 99% sequence similarity threshold was used for species-level identifications for all taxa; assignments to genus level were based on percent similarity and the BLAST taxonomy report.
Parasite prevalence, intensities and densities were defined according to Bush et al. (Reference Bush, Lafferty, Lotz and Shostak1997). Mean intensities could not be determined for the cyclophyllidean, the ciliates (apostome and sessilid), the gregarine or the microsporidian (Table 2). Prevalence only was recorded for the cyclophyllidean, gregarine and microsporidian. For the ciliates, relative abundance was calculated for the apostome and estimated for the sessilid (see below). Mean intensities and relative abundances are expressed as ± standard error (s.e.).
Table 2. Parasite taxa identifications, BLAST results and quantitative factors used in analyses. N/A = sequencing was unsuccessful or not done. P = prevalence. Only species with intensity (I) or relative abundance (RA) were included in NMDS and associated analyses.

a Gregarinasina is a subclass of Apicomplexa, but specimens could not be identified past this level of classification, and are consequently referenced only as ‘gregarines’.
b As of this publication, the GenBank record had not been updated to reflect the findings of Landers et al. (Reference Landers, Lee, Walters, Walker, Powell, Patel and Frischer2020) who associated this GenBank accession number with Hyalophysa lynni. Specimen ID agrees with organism name where BLAST results returned 99% similarity or higher, otherwise only genus level was confirmed.
c A single specimen was identified so it was included as part of the trypanorhynchs for the purpose of analyses.
Macroscopic assessment of black gill condition
A black gill score was determined macroscopically for individual shrimp by evaluating the darkest portion of gills in each specimen in the field using a colour standard scale comprised of increasingly pigmented paint swatches as follows based on the hexadecimal colour code: 1 = #DFD3C3, 2 = #DACAB2, 3 = #CCB79B, 4 = #C0A98B, 5 = #AB9579, 6 = #947F65, 7 = #6E543C, 8 = #5A4A3D, 9 = #564536 and 10 = #48423C. A black gill score from 1 (no melanization) to 10 (darkest melanization) was recorded for each specimen, with scores >5 categorizing individuals as shrimp with black gill and scores of ⩽5 categorizing individuals as shrimp without black gill.
Assessment of infection by gill parasites
To examine gill parasites (i.e., the sessilid and apostome ciliates), the 4 most posterior gill filaments of the haphazardly chosen left or right side were removed from each juvenile, subadult and adult shrimp (n = 543) and preserved in 3–5% formalin or frozen in deionized water until later examination (Martin et al., Reference Martin, Quintero, Quigley and Khosrovian2000). Wet mounts of the gill filaments were observed using light microscopy at 200× total magnification. Gill filaments were subdivided into 3 non-overlapping fields of view (FOVs) each of 0.785 mm2. Smaller gills typically allowed for only 1 or 2 FOVs per filament. The number of trophonts of the apostome ciliate in each FOV was recorded and relative abundance calculated as the number of apostome ciliates per mm2 of gill tissue. For sessilid ciliates, relative abundance was estimated according to the number of FOVs showing stalks of these parasites; this value was corrected for the number of FOVs available in an individual shrimp.
Statistical analyses
Parasite prevalence, intensities and abundances were used for statistical analyses, which were performed in R version 4.0.3 (R Core Team, 2019) (Table 2). As only 1 individual of the trypanorhynch Kotorella pronosoma (Stossich, 1901) was observed, it was combined with the other trypanorhynch (Prochristianella sp.) in the analyses. Due to low parasite infections in white shrimp post-larvae, statistical analyses were restricted to juvenile, subadult and adult life stages. When testing how individual parasites relate to the patterns of black gill prevalence, analyses were restricted to collections made from the open water localities during the black gill season (August through October).
To assess variability among parasite communities across ontogenetic and spatial factors (i.e., localities), parasite abundances were first averaged for each trawl and then standardized using the Wisconsin double standardization tool, which standardizes species by the maximum value and then standardizes localities by totals, using the ‘vegan’ package (Oksanen et al., Reference Oksanen, Blanchet, Friendly, Kindt, Legendre, McGlinn, Minchin, O'Hara, Simpson, Solymos, Stevens, Henry, Szoecs and Wagner2019). Bray–Curtis distance matrices of parasite abundances were then developed and used to create non-metric multidimensional scaling (NMDS) plots with 95% data ellipses to examine shrimp parasite communities among localities. Water temperature (°C), salinity (psu), shrimp length and black gill score were tested for correlation with multivariate distances using permutation tests with 999 iterations and visualized as vectors using the ‘envfit’ function in the ‘vegan’ package (Oksanen et al., Reference Oksanen, Blanchet, Friendly, Kindt, Legendre, McGlinn, Minchin, O'Hara, Simpson, Solymos, Stevens, Henry, Szoecs and Wagner2019). Parasite taxa names overlaid onto the ordination plot illustrate where taxa correspond to one another within the ordination space. Permutational analyses of variance (PERMANOVA) and corresponding pairwise post hoc tests using the ‘RVAideMemoire’ package (Hervé, Reference Hervé2021) were also conducted to examine specific differences among parasite communities of shrimp groups (i.e., juvenile vs subadult vs adult and Ashley River vs Wando River vs open water Charleston Harbor localities).
Indicator species analyses were performed using multi-level pattern analysis (‘multipatt’ function within the ‘indicspecies’ package; de Cáceres and Legendre, Reference De Cáceres and Legendre2009) to test for parasite taxa that were significantly associated with white shrimp life stage or locality. Indicator values (IV), i.e., the proportion of parasite x occurring in group A multiplied by the proportion of individuals in group A that contain parasite x (Dufrene and Legendre, Reference Dufrene and Legendre1997), were also calculated to determine how strongly a parasite grouped with a particular locality or host life stage.
Species-specific mixed-effects logistic regression analyses (‘glmer’ function within the ‘lme4’ package; Bates et al., Reference Bates, Maechler, Bolker and Walker2015), with collection event included as a random effect to account for the lack of independence within sampling events, were used to assess how parasite presence related to white shrimp life stage and collection locality. Model significance was assessed using a likelihood ratio test followed by Tukey's post hoc analyses to identify pairwise differences performed using the ‘multcomp’ package (Hothorn et al., Reference Hothorn, Bretz and Westfall2008). The relationship between the apostome ciliates and black gill from open water localities in fall months was tested using a binomial test and a mixed-effects segmented regression with collection event included as a random effect. Differences in parasite prevalence and intensity/relative abundance between shrimp with black gill and those without black gill were examined using mixed-effects approaches in the ‘lme4’ package (Bates et al., Reference Bates, Maechler, Bolker and Walker2015) followed by Tukey's comparisons of estimated marginal means in the ‘emmeans’ package (Lenth, Reference Lenth2022). These models were restricted to collections in open water habitats in fall months and for quantifiable parasites found in the Harbor during these months (i.e., apostomes, rhabditids, lecanicephalideans, trypanorhynchs and plagiorchiids). For these models, random factors of individual shrimp, collection month, sampling event, date and sampling event nested within date were included if they enhanced model performance by not contributing to model overfitting.
Results
Parasite community composition
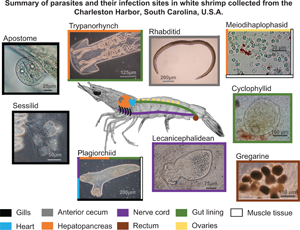
Parasites belonging to 10 genera of 6 classes were found to infect white shrimp in the Charleston Harbor watershed (Table 2; Fig. 2). Of the 597 white shrimp specimens examined (post-larvae to adult), 82% (491) were infected with at least 1 type of parasite. These parasites included 6 helminths [4 cestodes (1 lecanicephalidean, 1 cyclophyllidean and 2 trypanorhynchs), 1 plagiorchiid digenean and 1 rhabditid nematode], 2 ciliates (1 sessilid and 1 apostome), 1 gregarine and 1 microsporidian. All worms were found in a larval stage (i.e., metacercariae for the plagiorchiid, plerocerci for trypanorhynchs and juveniles for the rhabditid). Four of the parasite taxa were able to be identified to species-level using DNA sequencing (Table 2). Sequences from the nematode and 2 of the cestodes were only 96–97% similar to any sequence in GenBank and thus could only be identified to genus level (Table 2). Gregarine specimens could not be amplified or sequenced. Sequences from this study were deposited into GenBank under accession numbers OL456224–OL456226 and OL467311–OL467319 [platyhelminthes (n = 6), ciliate (n = 1), nematodes (n = 3) and microsporidians (n = 2)]. Parasites will hereafter be referenced as gregarines, apostomes, sessilids, microsporidians, rhabditids, cyclophyllids, lecanicephalideans, trypanorhynchs and plagiorchiids (Table 2).

Fig. 2. Parasites of the white shrimp, Penaeus setiferus observed in the Charleston Harbor watershed, South Carolina, USA. (A) Excysted metacercaria of the plagiorchiid Opecoeloides fimbriatus, scale bar 150 μm. (B) Juvenile of the rhabditid Hysterothylacium reliquens, scale bar 200 μm. (C) Microsporidian meiodihaplophasid Agmasoma penaei, scale bar 25 μm. (D) Sessilid Zoothamnium sp., scale bar 75 μm. (E) Cyclophyllidean Parorchites zederi, scale bar 85 μm. (F) Lecanicephalidean Polypocephalus sp., scale bar 75 μm. (G) Scolex of plerocercus of the trypanorhynch Prochristianella sp., scale bar 100 μm. (H) Gregarine gametocysts, scale bar 100 μm. (I) Apostome Hyalophysa lynni, scale bar 40 μm.
Ontogenetic and spatial variation in parasite communities
Prevalence and mean intensity or relative abundance of parasite infections varied across localities and white shrimp life stages (Table 3). Juvenile shrimp had significantly lower prevalence of infection by the rhabditid, lecanicephalidean and trypanorhynchs than adult shrimp (Table 4). Subadult shrimp had significantly higher prevalence of infection by the plagiorchiid and rhabditid than juvenile shrimp, but significantly lower prevalence of infection by the sessilid and lecanicephalidean than adult shrimp (Table 4). White shrimp collected at the open water Charleston Harbor localities had a higher prevalence of infection by the rhabditid, lecanicephalidean, trypanorhynchs and plagiorchiid, but lower prevalence of infection by the sessilid than those collected in the Ashley River and Wando River watersheds (the rhabditid was not collected from the Wando River; Tables 3 and 4). Parasite prevalence did not differ significantly between the Ashley River and Wando River watersheds (Tables 3 and 4).
Table 3. Prevalence (%)/mean intensity or relative abundance (±s.e.) of black gill and parasite infections by shrimp life stage and by locality

a See Materials and methods: shrimp gill examination and ciliate abundance for differences in abundance calculations for these 2 ciliate parasites.
Table 4. Pairwise P values from Tukey's post hoc tests of logistic regression models that examined parasite presence across shrimp life stages and localities (n = 532). Shrimp life stage and locality were significant factors (P < 0.01) in their respective models based on likelihood ratio tests unless indicated by ‘n.s.’ for not significant, in which case pairwise analyses were not conducted.

Significance at P < 0.05 of pairwise tests is indicated in bold font.
Parasite community structure varied significantly among both white shrimp life stages (PERMANOVA, n = 65, R 2 = 0.24, P = 0.001) and localities (PERMANOVA, n = 52, R 2 = 0.31, P = 0.001). The parasite communities of juveniles were significantly different from those of both adults and subadults (P = 0.002), which were not significantly different from each other (P = 0.233). Parasite community dissimilarities were also significantly correlated with black gill score (R 2 = 0.29, P = 0.002), shrimp length (R 2 = 0.41, P = 0.001), salinity (R 2 = 0.16, P = 0.022), but not water temperature (R 2 = 0.11, P = 0.068; Fig. 3). The apostome and the black gill score vectors were both located at higher y-axis values, but trypanorhynchs and plagiorchiids were located at lower y-axis values, highlighting that these factors were associated with different communities of parasites (Fig. 3).

Fig. 3. Non-metric multi-dimensional scaling (NMDS) plot of white shrimp infected with parasites in the greater Charleston Harbor watershed, South Carolina, USA (stress = 0.11). Data are presented by collection localities: Ashley River (filled black circles), Wando River (open circles) and Charleston Harbor (filled grey circles). Vectors represent significant (P ⩽ 0.01) factors related to community structure; taxa names (e.g. Sessilid) highlight locations in ordinal space most associated with those taxa. Dashed ovals represent data ellipses for parasite communities from each locality.
Significant indicators of white shrimp collected at open water Charleston Harbor localities included the rhabditid (IV = 0.998, P = 0.001), plagiorchiid (IV = 0.907, P = 0.001), apostome (IV = 0.868, P = 0.001), trypanorhynch (IV = 0.855, P = 0.001) and lecanicephalidean (IV = 0.772, P = 0.001), while the lecanicephalidean (IV = 0.852, P = 0.005) and rhabditid (IV = 0.799, P = 0.005) were significant indicators of adult white shrimp. The sessilid was a significant indicator of juvenile white shrimp (IV = 0.355; P = 0.005) and of shrimp collected in the Ashley River watershed (IV = 0.767; P = 0.001).
Parasite communities and black gill
No post-larval white shrimp exhibited macroscopic evidence of black gill, which was almost exclusively found in specimens collected at open water Charleston Harbor localities, regardless of shrimp life stage. Black gill score was a significant vector associated with parasite community structure (Fig. 3). For shrimp collected in open water habitats in the fall, parasite communities between shrimp with and without black were significantly different (PERMANOVA, n = 9, R 2 = 0.31, P = 0.008). Indicator species analyses showed that only the apostome was a significant indicator of shrimp with black gill (IV = 0.93; P = 0.005). The apostome was observed across 3 localities (Table 3). Juvenile, subadult and adult white shrimp were all observed with both black gill and apostome infections (Table 3), but significantly more adult white shrimp were infected with the apostome ciliate than exhibited black gill (binomial test P < 0.001). A positive slope existed between black gill scores ⩾6 (modeled breakpoint = 5.68 and the relative abundance of the apostome on the gills of white shrimp collected during the black gill season (slope = 0.83, confidence interval = 0.35 – 1.3), but there was no significant relationship between these 2 variables for scores of ⩽5 (slope = 0.025, confidence interval = −0.47 – 0.35, Fig. 4).

Fig. 4. Black gill score related to apostome ciliate Hyalophysa lynni abundance for individual white shrimp (filled grey circles) and mean abundances (filled black circles ± s.e.; n = 306). Segmented regression showed a significant relationship between black gill score (when ⩾6) and apostome abundance on individual shrimp.
Generalized mixed-effects models on prevalence showed parasite (χ 2 = 275.9, P < 0.001) and black gill (χ 2 = 24.8, P < 0.001) as significant factors, with individual shrimp (s.d. = 0.341) and collection month (s.d. = 0.211) included as random effects to account for sampling design. Post hoc analysis showed prevalence of infection by the trypanorhynchs, and with marginal significance of the rhabditid, was higher in shrimp without black gill than those with this condition (Table 5). The opposite occurred for the apostome, for which prevalence was higher in shrimp with black gill (Table 5; Fig. 5A). Generalized mixed-effects models on intensity showed parasite (χ 2 = 20.8, P = 0.008) and black gill (χ 2 = 22.1, P < 0.001) as significant factors, with individual shrimp (s.d. = 0.212) and collection month (s.d. = 0.001) included as random effects to account for sampling design. Post hoc analysis showed mean intensities of the rhabditid, and with marginal significance of the trypanorhynchs, were higher in shrimp without black gill than in those with this condition (Table 5; Fig. 5B). This pattern was again reversed for the apostome (Table 5; Fig. 5B). There was no significant association between the presence or intensity of the lecanicephalidean or plagiorchiid and black gill status.

Fig. 5. Infection metrics of parasites in white shrimp with black gill (black) and without black gill (white). Prevalence presented as (A) the percentage of shrimp infected and (B) mean intensity (±s.e.) and mean relative abundance (±s.e.) (for the apostome). Asterisks indicate significant differences between groups for prevalence (logistic regression, Table 5) and mean intensity or mean relative abundance (general linear mixed-effects model, Table 5).
Table 5. Marginal means pairwise contrasts from mixed-effects models of parasite prevalence or intensity in shrimp with and without black gill

Significance at P < 0.05 is indicated with bold font.
Discussion
The analyses presented here demonstrate differences in the parasite communities of white shrimp that were associated with localities (i.e., tidal creek vs open water habitats), host life stages and the occurrence of black gill. Juvenile white shrimp generally live in tidal creeks until they reach a certain size or level of maturity at which time they egress towards more saline waters in the summer and fall (Lindner and Cook, Reference Lindner and Cook1967). While the habitat and life stage of white shrimp were both significantly related to the composition of the parasite community in this study, the confounding nature of these variables makes it difficult to tease apart the individual effects of these factors. Despite this limitation, this study allowed for a deeper understanding of the ontogenetic and spatial dynamics of white shrimp parasite community composition, as well as the potential physiological impacts of parasites on this economically and ecologically important shrimp species.
To our knowledge, the only previous helminth survey of white shrimp in the Georgia Bight was limited to a total of 29 specimens and reported infection by ‘cestodes, trematodes and nematodes’ (Tripp and Turner, Reference Tripp and Turner1983). All parasites encountered herein have previously been reported from penaeid shrimp, including white shrimp in the Gulf of Mexico (Overstreet, Reference Overstreet1973; Couch, Reference Couch1978; Deardorff and Overstreet, Reference Deardorff and Overstreet1981; Fontaine, Reference Fontaine1985; Carreon et al., Reference Carreon, Faulkes and Fredensborg2018), with the exception of the apostome H. lynni, which was only recently described from white shrimp from the South Atlantic Bight (Landers et al., Reference Landers, Lee, Walters, Walker, Powell, Patel and Frischer2020). An unidentified apostome ciliate on shrimp gills was associated with black gill in the Gulf of Mexico, albeit prior to the description of H. lynni (Overstreet, Reference Overstreet1973; Couch, Reference Couch1978).
In host–parasite systems, the diversity of parasites often changes ontogenetically with habitat and diet (Muñoz and Zamora, Reference Muñoz and Zamora2011; Münster et al., Reference Münster, Klimpel, Fock, MacKenzie and Kuhn2015). As such, higher prevalence and mean intensities of infection by the rhabditid, trypanorhynchs and lecanicephalidean in subadult and adult white shrimp are potentially attributable to the nature of the life cycles of these parasites. These 3 parasites infect white shrimp via their ingestion of either free-living stages or infected planktonic hosts (Johnson, Reference Johnson1975; Carreon et al., Reference Carreon, Faulkes and Fredensborg2018). Thus, as shrimp age, opportunities for infection by these parasites increase and parasites can accumulate in their hosts.
The apostome ciliate was not found in post-larval white shrimp but it did infect all other life stages and was found across localities. Frischer et al. (Reference Frischer, Lee, Price, Walters, Bassette, Verdiyev, Torris, Bulski, Geer, Powell, Walker and Landers2017) found correlations between the presence of this ciliate and black gill suggesting a linear response of the degree of gill melanization to the level of apostome infection. Results herein confirm such a relationship; however, it does not appear linear according to our analyses as many individuals infected with this ciliate did not exhibit black gill. Our data also indicate that a H. lynni abundance threshold may exist whereby only high abundances are associated with gill melanization (i.e., black gill). A delay between infection and melanization of the gill tissue, or the host response eliminating suitable parasite habitat leading to no infection at extreme levels of melanization (sensu Frischer et al., Reference Frischer, Lee, Price, Walters, Bassette, Verdiyev, Torris, Bulski, Geer, Powell, Walker and Landers2017) could also contribute to the non-linearity of this relationship.
Black gill is indicative of an immune response (Lightner and Redman, Reference Lightner and Redman1977; Martin et al., Reference Martin, Quintero, Quigley and Khosrovian2000; Cerenius et al., Reference Cerenius, Kawabata, Lee, Nonaka and Soderhall2010) that can generate suboptimal living conditions for endoparasites (Burnett and Burnett, Reference Burnett and Burnett2015) given its negative impact on the physiology of the hosts. Hence, activated immune response in shrimp with black gill could lead to the observed reduced prevalence and/or intensities of trypanorhynchs and rhabditids in white shrimp with black gill compared to those without black gill. Alternatively, given the role of the hepatopancreas in the crustacean immune system (Rőszer, Reference Rőszer2014; Cao et al., Reference Cao, Pan, Sun, Liu and Lan2021), damage caused by trypanorhynchs, which embed deeply in this organ and alter its function in portunid crabs (Gurney et al., Reference Gurney, Johnston and Nowak2006), could lead to immunosuppression. This could explain significantly higher prevalence of infection of white shrimp without black gill and immunosuppression could facilitate infection by other parasites, including the rhabditid that was also found in higher intensities in shrimp without black gill. Thus, while the apostome ciliate H. lynni remains a significant factor associated with black gill outbreaks and the degree of gill melanization in individual white shrimp, other parasites such as the trypanorhynchs and rhabditids may also influence the patterns of black gill. To further support the role of parasitic co-infection in black gill occurrence, analyses of fisheries-independent data show no significant relationship between black gill prevalence and white shrimp abundance (Kendrick et al., Reference Kendrick, Brunson, Frischer and Kingsley-Smith2021). Environmental factors, such as salinity and temperature, also have the potential to influence both patterns of black gill (Fowler et al., Reference Fowler, Leffler, Johnson, DeLancey and Sanger2018; Swinford and Anderson, Reference Swinford and Anderson2021) as well as parasite communities (Koprivnikar et al., Reference Koprivnikar, Ellis, Shim and Forbes2014; Strepparava et al., Reference Strepparava, Segner, Ros, Hartikainen, Schmidt-Posthaus and Wahli2018), such that future studies should investigate the potential interrelatedness of biotic and abiotic factors in determining white shrimp health metrics.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182022001597
Data availability
The data that support the findings of this study are available from the corresponding author, MRK, upon reasonable request.
Acknowledgements
The authors thank Dr Robert D. Podolsky for his comments on an earlier draft of this manuscript, as well as Stephen Czwartacki, Kristin Hamilton, Elizabeth Gooding and Elizabeth Underwood for their field assistance. The authors also thank Pam Corwin (SCDNR) for providing the shrimp artwork for the graphical abstract. This publication represents SCDNR Marine Resources Research Institute Contribution Number 860.
Author's contributions
S. R. Z. collected the data and performed the analyses. I. d. B. co-conceived and designed the analysis, collected the data and contributed to analyses. P. R. K.-S. contributed to the design of the analyses. K. M. H.-S. collected the data and contributed to data analysis. N. F. assisted with the processing of specimens in the laboratory. M. R. K. co-conceived and designed the analysis, collected the data, contributed to, and performed, the analyses. All authors contributed to the writing and editing of the article.
Financial support
This study was supported by the South Carolina Saltwater Recreational Fishing License Fund and Award# NA14OAR4170088 from South Carolina Sea Grant Consortium.
Conflict of interest
None.
Ethical standards
Not applicable.