Introduction
Nest building behaviour is widespread across avian species, being a critical stage that may influence the success or failure of reproduction (Mainwaring et al., Reference Mainwaring, Stoddard, Barber, Deeming and Hauber2023). Animals invest considerable time and effort in constructing nests that regulate temperature and humidity (Korb and Linsenmair, Reference Korb and Linsenmair1999), and provide shelter (Hansell, Reference Hansell2000) and protection for their offspring (Hansell, Reference Hansell2005). Nesting behaviour encompasses more than mere construction, as it usually involves the strategic incorporation of additional materials, such as hair, feathers or plants, which are not essential for the nest structure but may maximize reproductive success (Bush and Clayton, Reference Bush and Clayton2018). However, while the significance of nest structure and location has been extensively studied (Hansell, Reference Hansell2005; Mainwaring et al., Reference Mainwaring, Hartley, Lambrechts and Deeming2014, Reference Mainwaring, Stoddard, Barber, Deeming and Hauber2023), the ecological role of additional nest materials, particularly those of plant origin, remains less explored.
Secondary plant fragments (hereafter ‘secondary plants’ for simplicity) that are deposited on the nest by some bird species represent a tiny percentage of the nest material (i.e. 4–13%; see Petit et al., Reference Petit, Hossaert-McKey, Perret, Blondel and Lambrechts2002 in the blue tit Cyanistes caeruleus; Clark and Mason, Reference Clark and Mason1985, in European starling Sturnus vulgaris; and Dykstra et al., Reference Dykstra, Hays and Simon2009 in the red-shouldered hawk Buteo lineatus). Additionally, plant fragments are usually deposited on the nest cup or rim. Thus, they are in direct contact with the clutch, the incubating parent or the nestlings, and are clearly visible for the attending parents. Depending on the species, only males (e.g. European and spotless starling, Sturnus unicolor; Clark and Manson, Reference Clark and Mason1985; Veiga et al., Reference Veiga, Polo and Viñuela2006), only females (e.g. blue tits, Petit et al., Reference Petit, Hossaert-McKey, Perret, Blondel and Lambrechts2002), or both parents (e.g. broad-winged hawk, Buteo platypterus, Lyons et al., Reference Lyons, Titus and Mosher1986; McCabe et al., Reference McCabe, Goodrich, Master and Bordner2019) provide secondary plants to their nests. For example, starling males build a nest mainly done with tree branches that they typically cover with a collection of green plants, hair and feathers (Gwinner, Reference Gwinner1997; Veiga et al., Reference Veiga, Polo and Viñuela2006).
Historically, three non-mutually exclusive hypotheses have been proposed to explain why some bird species incorporate secondary plants to their nests. First, the ‘Courtship hypothesis’ holds that plant fragments function as a secondary sexual trait. For example, carrying of plants by starling males is synchronized with the females’ fertile period, presumably to stimulate them to breed (Gwinner, Reference Gwinner1997; Gwinner et al., Reference Gwinner, Oltrogge, Trost and Nienaber2000; Brouwer and Komdeur, Reference Brouwer and Komdeur2004; Polo et al., Reference Polo, Veiga, Cordero, Viñuela and Monaghan2004; Veiga et al., Reference Veiga, Polo and Viñuela2006). In addition, carrying of secondary plants and nest size in blue tits has been suggested as sexual signals of the females to stimulate differential allocation in males (Gwinner, Reference Gwinner1997; Tomás et al., Reference Tomás, Merino, Martínez-de La Puente, Moreno, Morales and Rivero-De Aguilar2013; Gwinner et al., Reference Gwinner, Oltrogge, Trost and Nienaber2000). The other two hypotheses are related to the fact that most secondary plants are aromatic and possess specific properties that enhance anti-parasite defences in birds. This might be particularly important in the nest environment of altricial birds due to the accumulation of nest materials, organic compounds and to a relatively high temperature, all of which favours the presence of nest parasites and blood-parasite vectors (Mainwaring et al., Reference Mainwaring, Hartley, Lambrechts and Deeming2014; Scott-Baumann and Morgan, Reference Scott-Baumann and Morgan2015).
Specifically, the ‘Nest protection hypothesis’ posits that plant metabolites, including volatile compounds, would reduce the load of nest parasites and blood-parasite vectors (Clark and Mason, Reference Clark and Mason1985;, Reference Clark and Mason1988; Clark, Reference Clark, Loye and Zuk1991; Dubiec et al., Reference Dubiec, Góźdź and Mazgajski2013; Scott-Baumann and Morgan, Reference Scott-Baumann and Morgan2015). On the other hand, the ‘Drug hypothesis’ proposes that secondary plants directly promote offspring health and development via e.g. stimulation of the immune system (Gwinner et al., Reference Gwinner, Oltrogge, Trost and Nienaber2000), which allows individuals to better cope with parasites. Certainly, there is evidence that secondary plants constitute the first anti-parasite barrier, as their occurrence is negatively associated with the presence of blood-parasite vectors (Lafuma et al., Reference Lafuma, Lambrechts and Raymond2001; Gwinner and Berger, Reference Gwinner and Berger2006), nest-dwelling ectoparasites (Clark and Mason, Reference Clark and Mason1988; Tomás et al., Reference Tomás, Merino, Martínez-de la Puente, Moreno, Morales, Lobato and del Cerro2012) and bacteria (Mennerat et al., Reference Mennerat, Mirleau, Blondel, Perret, Lambrechts and Heeb2009). For example, the experimental addition of aromatic plants in blue tit nests, and particularly, of Lavandula stoechas, had a strong repellent effect against the mosquito Culex pipiens, and this and other plants such as Achillea ligustica had a masking effect on the chemical cues used by mosquitoes to find their hosts (Lafuma et al., Reference Lafuma, Lambrechts and Raymond2001).
Given the severe consequences of nest parasites for nestling growth and survival (Hurtrez-Boussès et al., Reference Hurtrez-Boussès, Perret, Renaud and Blondel1997; Simon et al., Reference Simon, Thomas, Blondel, Perret and Lambrechts2004; Thomas et al., Reference Thomas, Shipley, Blondel, Perret, Simon and Lambrechts2007), various studies have focussed on exploring the effects of secondary plants on the nestlings. Indeed, in different bird species, the occurrence of secondary plants is associated with enhanced chick growth (Gwinner et al., Reference Gwinner, Oltrogge, Trost and Nienaber2000; Pires et al., Reference Pires, Belo, Diamantino, Rabaça and Merino2020) and physiological condition (e.g. higher blood haemoglobin levels, Clark and Mason, Reference Clark and Mason1988; Glądalski et al., Reference Glądalski, Bańbura, Kaliński, Markowski, Skwarska, Wawrzyniak, Zieliński and Bańbura2020; higher haematocrit levels, Gwinner et al., Reference Gwinner, Oltrogge, Trost and Nienaber2000, Mennerat et al., Reference Mennerat, Mirleau, Blondel, Perret, Lambrechts and Heeb2009; longer telomere length, Soler et al., Reference Soler, Ruiz-Castellano, Figuerola, Martín-Vivaldi, Martínez-de la Puente, Ruiz-Rodríguez and Tomás2017), which supports both the ‘Nest protection hypothesis’ and the ‘Drug hypothesis’. However, not only the nestlings, but also the parents and, particularly, the mothers are highly exposed to nest parasites and blood-parasite vectors, given that they spend quite a considerable amount of time in the nest during construction, incubation and nestling provisioning. Accordingly, it has been observed that female great tits (Parus major) avoid sleeping in nests that have been experimentally infested with fleas (Christe et al., Reference Christe, Richner and Oppliger1996). Furthermore, the exposure to blood-parasite vectors (i.e. biting midges) imposes high physiological costs on females (e.g. see Martínez-de la Puente et al., Reference Martínez-de La Puente, Merino, Tomás, Moreno, Morales, Lobato and Martínez2011 in the blue tit). However, although previous evidence suggest that blue tit females are selective in provisioning plants (Petit et al., Reference Petit, Hossaert-McKey, Perret, Blondel and Lambrechts2002; Pires et al., Reference Pires, Belo and Rabaça2012; Garrido-Bautista et al., Reference Garrido-Bautista, Ramos, Arce, Melero-Romero, Ferreira, Santos-Baena and Norte2023), the link between secondary plants and blood-parasite infection in adult birds remains unexplored.
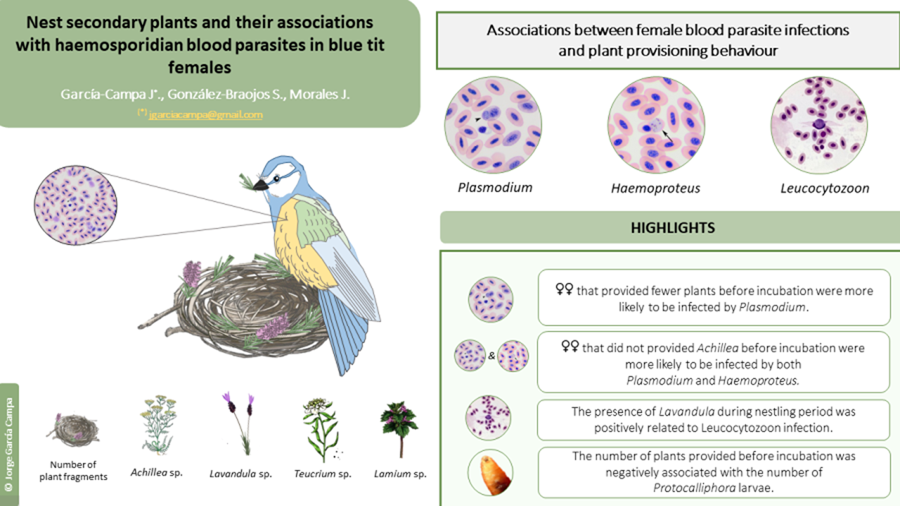
The aim of this study was twofold. First, we investigated whether the blood-parasite infection (i.e. presence/absence of Plasmodium, Haemoproteus, and Leucocytozoon) of blue tit females was associated with the occurrence of particular plant genera in the nest during different stages of reproduction. Second, we explored the link between the different plant genera and the occurrence of two common nest-dwelling ectoparasites (i.e. blowfly pupae Protocalliphora azurea and mites Dermanyssus gallinoides). In the blue tit, females build the nest alone and show a marked preference for incorporating secondary plants in their nests. The provisioning of secondary plants involves a small percentage of the plant species available in the habitat (e.g. 5% in blue tits; Petit et al., Reference Petit, Hossaert-McKey, Perret, Blondel and Lambrechts2002; Pires et al., Reference Pires, Belo and Rabaça2012; Dubiec et al., Reference Dubiec, Góźdź and Mazgajski2013), suggesting that females are very selective and show a preference for particular plants. Most plant species gathered by females are aromatic (e.g. Achillea sp., Lavandula sp.; Pirmohammadi et al., Reference Pirmohammadi, Shayeghi, Vatandoost, Abaei, Mohammadi, Bagheri, Khoobdel, Bakhshi, Pirmohammadi and Tavassoli2016; Caputo et al., Reference Caputo, Reguilon, Mińarro, De Feo and Rodriguez-Arias2018) and characterised by a high content of secondary metabolites, including volatile chemicals known for their anti-parasitic and anti-pathogenic properties (Hart, Reference Hart, Clayton and Moore1997; see also Ruiz-Castellano et al., Reference Ruiz-Castellano, Tomás, Ruiz-Rodríguez, Martín-Gálvez and Soler2016 in spotless starling).
We predicted that females that provide more plant fragments to their nests are less likely to show blood-parasite infection, given that secondary plants can repel blood-parasite vectors (‘Nest-protection hypothesis’) and can also directly promote immune responsiveness and condition (‘Drug hypothesis’ as applied to adult birds). In particular, we expected that blood-parasite prevalence should be more strongly associated with plant provisioning behaviour at early stages of reproduction rather than during the nestling period, given that females spend more time in the nest (and are thus more exposed to blood-parasite vectors) during egg laying and incubation. Similarly, we expected that the provisioning of secondary plants would reduce the development of nest-dwelling ectoparasites.
Material and methods
Study area and model species
The study was carried out in the locality of Miraflores de la Sierra, Madrid, central Spain (40°48′N, 03°47′W) throughout the breeding season of 2017. We studied a blue tit population breeding in nest-boxes in a deciduous forest at 1200 m a.s.l., mainly dominated by Pyrenean oak (Quercus pyrenaica). The blue tit is a socially monogamous passerine with intense bi-parental care. However, nest building is done by the female. The nests are built with moss and are lined with soft material such as hair and feathers. Additionally, blue tit females in the study population usually include pieces of other green plants, which they mostly incorporate when the nest is almost finished, and during egg laying and incubation of the eggs (Bańbura et al., Reference Bańbura, Blondel, de Wilde-Lambrechts and Perret1995; Mainwaring, Reference Mainwaring2017). However, unlike other species like starlings, the provisioning of secondary plants continues during the whole breeding process (i.e. from the end of nest construction until fledging; Mennerat et al., Reference Mennerat, Mirleau, Blondel, Perret, Lambrechts and Heeb2009). The blue tit has one of the largest clutch sizes and the largest variation in clutch size among all European passerines (in our study population; mean ± s.d.: 9.6 ± 1.8 eggs; n = 464 clutches; range 4–15). The incubation stage ranges between 14 and 16 days (von Haartmann Reference von Haartman1969; Salvador, Reference Salvador, Salvador and Morales2016).
General procedures
At the beginning of the breeding season, we started visiting nest-boxes to record the onset of nest construction. Once nest construction was at an advanced stage, we inspected the nests every other day when possible (mean ± s.d. = 11.27 ± 2.91 inspections; n = 110 nests) in order to study the occurrence of secondary plants (see Identification and quantification of secondary plants). We also recorded the laying date, the onset of incubation and the hatching date (=day 0).
At days 9–12, we trapped blue tit females in their nest-boxes, weighted them using a spring balance (accuracy 0.01 g), and collected a blood sample from the brachial vein with a sterilized needle and a heparinized capillary. Then, we made a blood smear for blood parasite identification (see Identification of blood parasites). Blood smears were air-dried and fixed on the same day using absolute methanol for 1 min. After such fixation, they were stained for 45 min with Giemsa diluted 1:10 in buffer, pH 7.2 (Schall, Reference Schall1986).
After fledging (i.e. day 20 approx.), all the nest material was collected in a sealed labelled plastic bag and kept at 4°C. Once in the laboratory, each nest was crumbled by hand on white paper to assess the presence/absence of two main nest dwelling parasites in blue tit nests: blowfly pupae of the dipteran P. azurea, which were also counted, and mites D. gallinoides. Additionally, we searched for all the dry plant fragments that remained in the nest after reproduction and weighed them with a high-precision balance (accuracy 0.001 g).
During the breeding season, we performed a parallel study, in which we placed feeders inside nest-boxes to supplement females with carotenoids (mainly lutein mixed with fat) prior and during egg laying (for details, see García-Campa et al., Reference García-Campa, Müller, González-Braojos, García-Juárez and Morales2020 and Supplementary Material). The experimental treatment (lutein-supplemented and control) was initially controlled for in all statistical analyses, but since it was never significant (all P > 0.10), we excluded it from the final models (see Table S2).
Identification and quantification of secondary plants
We recorded the provisioning of secondary plants at three stages of reproduction: before incubation (i.e. from the end of nest building to the onset of incubation; mean ± s.d. = 7.16 ± 2.53 visits), during incubation (i.e. from the onset of incubation to the hatching day; 2.61 ± 1.07 visits), and during the nestling period (i.e. from the hatching day to fledging; 1.51 ± 0.70 visits). At each visit, the different pieces of secondary plants found in the nest cup were placed on a white sheet of white paper, and were photographed together with a reference of 1 cm (1 picture per nest) for later count and identification in the laboratory. Plant fragments were placed back on the nest immediately after photographing them. In the field, it was not possible to weigh them accurately with portable balances, since each fragment usually weights less than 0.05 g. Thus, as mentioned above, we weighted them dry after nest collection, and once in the lab with a high precision balance (accuracy 0.001 g). We then used the photographs to count the total number of fragments found in each visit, regardless of plan genera. In addition, when possible, each fragment was assigned to a plant genus using a dichotomous key (General key to the Iberian flora – http://www.floraiberica.org/). However, identification of all fragments was not possible in some nests (26.9% before incubation, 20.9% during incubation, and 63.3% during the nestling period) and thus we decided to use the presence/absence of each plant genera instead of the number of fragments per genera.
To summarize, for each nest, we calculated the following three variables:
i) Mean number of fragments (i.e. total number of fragments, regardless of the plant genera, found in each visit divided by the number of visits made), separately for the three periods (before incubation, incubation and nestling stage).
ii) Presence/absence of particular plant genera, separately for the three periods (before incubation, incubation and nestling stage).
iii) ‘Final dry mass’ (i.e. total dry mass of all the plant fragments extracted from the nest material after fledging).
Identification of blood parasites
A half of each blood smear was scanned under 200× magnification in search of the extra-erythrocytic parasite Trypanosoma and the large intracellular Leucocytozoon However, only 3% of the females were infected by Trypanosoma and thus we did not analyse infection status by this parasite. In the other half of the blood smear, we scanned 50 fields under 1000× magnification using oil immersion, in search of intracellular parasites such as Plasmodium and Haemoproteus spp (see Merino et al., Reference Merino, Potti and Fargallo1997; Morales et al., Reference Morales, Moreno, Lobato, Merino, Tomás, Martínez De La Puente and Martínez2006).
Sample size and statistical analyses
We observed the provisioning of secondary plants in 110 blue tit nests. However, we could identify blood parasites in 67 females, given that some nests failed after laying or because females could not be trapped or blood sampled. The presence/absence of nest-dwelling ectoparasites (i.e. Protocalliphora and Dermanyssus) was assessed in 65 nests, since two of the nests failed in the second week after hatching and were thus not comparable with the rest.
We used R 4.1.0 (R Core Team 2020) for statistical analyses. We investigated whether the presence/absence of each blood parasite (i.e. Plasmodium, Haemoproteus and Leucocytozoon) and of each nest-dwelling ectoparasite (i.e. the presence/absence and the abundance of Protocalliphora and the presence/absence of Dermanyssus), was related with the (i) mean number of secondary plant fragments, (ii) presence/absence of particular plant genera, and (iii) final dry mass of secondary plants, during the three different stages (i.e. before incubation, incubation and nestling). For this, we performed separate generalized linear models for the presence/absence of each parasite, which was included as the dependent variable, using binomial distribution and logit link function. We ran different models for each variable describing the plant provisioning behaviour (see above i, ii and iii), which was included as a covariate. Additionally, for the number of blowflies, we run generalized linear models with negative binomial distribution. In all models, we additionally included the female's body mass (g) and the laying date (Julian calendar) as covariates to control for female condition and possible effects of reproductive phenology.
Results
Provisioning behaviour of secondary plants
During the whole reproductive period, we found at least one plant fragment in 103 out of the 110 nests that we followed (95.4% of nests). Separately, for each stage, we found at least one plant fragment in 58.5% of nests before incubation, 57.7% during incubation, and 88.1% during the nestling period. Similarly, for the subset of nests with female's blood parasite information, we found at least one plant fragment in 65 out of 67 nests (97.0% of nests). For each stage, in this subset of nests, we found at least one plant fragment in 58.2% of nests before incubation, 61.2% during incubation, and 92.4% during the nestling period.
We identified seven plant genera: Lavandula sp. (at least once in 67.2% nests), Anthriscus sp. (49.3%), Thymus sp. (35.8%), Achillea sp. (31.3%), Teucrium sp. (26.9%), Lamium sp. (23.9%) and Clinopodium sp. (13.4%). The presence of Lavandula sp. clearly increased during reproduction, being more than double throughout the nestling period than before incubation, while the ocurrence of Achillea sp. and Teucrium sp. remained more or less stable (Table 1). Anthriscus sp. was reduced during incubation, while Lamium sp. decreased throughout reproduction. Thymus sp. was only abundant during the nestling period and thus we did not analyse its presence at prior stages (Table 1). Finally, Clinopodium sp. was very scarce and we did not include it in further analyses (Table 1).
Table 1. Provisioning behaviour of secondary plant species

Percentages of appearance of the 7 plan genera (Lavandula sp., Anthriscus sp., Thymus sp., Achillea sp., Teucrium sp., Lamium sp., and Clinopodium sp.) found in blue tit nests during the different periods: before the incubation, during the incubation, nestling period and total. Significant associations between periods are marked with an asterisk (* for P < 0.05 and *** for P < 0.001. P values were obtained from chi square tests). For detailed statistics, see Supplementary Table S1.
Association between blood parasites and secondary plants
Fifty out of 67 females (74.63%) were infected by at least one blood parasite (38 females by Plasmodium, 42 by Haemoproteus and 15 by Leucocytozoon). Regardless of the plant genera, the presence/absence of Plasmodium was associated with the mean number of plant fragments before incubation, but not at other reproductive stages (Table S2). Specifically, females that provided on average more plant fragments before incubation were less likely to be infected by Plasmodium later on (Table S2; Figure 1a). The presence/absence of Haemoproteus or Leucocytozoon was not associated with the mean number of fragments at any reproductive stage (Table S2), although females that provided more plant fragments before incubation were marginally (and non-significantly) less likely to be infected by Haemoproteus (Table S2; Figure 2a). Female body mass or laying date were not associated with the presence/absence of any of the blood parasites (all P > 0.25 for female mass and all P > 0.07 for laying date; Table S2; see also Table S3).

Figure 1. Relationship between the prevalence of Plasmodium infection in blue tit females with (a) the mean number of plant fragments before incubation, and (b) the presence of Achillea sp. fragments before incubation. Values are means ± SE.

Figure 2. Relationship between the prevalence of Haemoproteus infection in blue tit females with (a) the mean number of plant fragments before incubation, and (b) the presence of Achillea sp. herbs before incubation. Values are means ± SE.
When analysing the presence/absence of the different plant genera and their association with blood parasites, we found that females were less likely to be infected by Plasmodium when they had included Achillea sp. in the nest before incubation (Table 2a; Figure 1b). This association remained significant throughout incubation (Table 2b), but not in the nestling period (Table 2c). Infection by Plasmodium was not associated with the occurrence of other plant genera (Table 2). Similarly, females were less likely to be infected by Haemoproteus when they had included Achillea sp. before incubation (Table 2a; Figure 2b). Furthermore, females that provided Lavandula sp. fragments during incubation were also less likely to be infected by Haemoproteus, although in this case the relationship was marginally non-significant (Table 2b). On the contrary, females were more likely to be infected by Leucocytozoon when they had included Teucrium sp. fragments in the nests before incubation (Table 2a; Figure 3a), and Lavandula sp. fragments during the nestling period (Table 2c; Figure 3b). The rest of associations was not significant (Table 2).
Table 2. Generalized linear models (GLMs) with binomial error distribution showing the relationships between the presence/absence of particular plant genera during 3 reproductive stages and (i) the females’ haemosporidian infection status (i.e. Plasmodium, Haemoproteus and Leucocytozoon) and (ii) nest-dwelling ectoparasites (i.e. Protocalliphora and Dermanyssus)

Thymus sp. is only included for the nestling period due to the low sample size before hatching. Significant effects (P < 0.05) are marked in bold. Whole models including female body mass and laying date are shown in Table S3 and S4.

Figure 3. Relationship between the prevalence of Leucocytozoon infection in blue tit females with the presence of (a) Teucrium sp. herbs before incubation, and (b) Lavandula sp. herbs during the nestling period. Values are means ± SE.
Association between ectoparasites and secondary plants
We found Protocalliphora pupae in 37 out of the 65 nests (56.9% of nests with female's parasite status). Interestingly, the abundance of Protocalliphora pupae was associated with the mean number of plant fragments before incubation (Table S2), but not at other reproductive stages (Table S2). Mites were present in 17 out of the 65 nests (26.1%), and their occurrence was not associated with the mean number of plant fragments provided (Table S2).
When we analysed the occurrence of particular plant genera, nests in which females provided Lamium sp. herbs before incubation were less likely to be infected by Protocalliphora pupae (Table 2a; Figure 4a). However, the presence of Dermanyssus was not related with the presence/absence of Lamium sp. (Table 2; Figure 4b) or with any other plan genus (Table 2).

Figure 4. Relationship between the presence of (a) Procalliphora azurea pupae and (b) Dermanyssus gallinoides with the presence of Lamium sp. fragments before the incubation period. Values are means ± SE.
Discussion
In this study, we investigated whether the provisioning behaviour of secondary plants in the blue tit, a bird species in which females alone build the nest, is associated with the female's blood-parasite infection and with the presence of ectoparasites in the nest. Historically, studies on the role of secondary plants in avian nests have focussed mainly on their antimicrobial and insect-repellent properties (Lafuma et al., Reference Lafuma, Lambrechts and Raymond2001; Tomás et al., Reference Tomás, Merino, Martínez-de la Puente, Moreno, Morales, Lobato and del Cerro2012; Ruiz-Castellano et al., Reference Ruiz-Castellano, Tomás, Ruiz-Rodríguez, Martín-Gálvez and Soler2016), and thus on their potential beneficial effect on the nestlings (Gwinner et al., Reference Gwinner, Oltrogge, Trost and Nienaber2000; Soler et al., Reference Soler, Ruiz-Castellano, Figuerola, Martín-Vivaldi, Martínez-de la Puente, Ruiz-Rodríguez and Tomás2017; Gwinner et al., Reference Gwinner, Capilla-Lasheras, Cooper and Helm2018). However, no study to date has investigated the association between secondary plants and the female's blood-parasite status.
As hypothesized, we found certain evidence for the above association, although it varied among the three blood parasites studied and across the different stages of reproduction. On the one hand, the prevalence of Haemoproteus and Leucocytozoon was apparently not related to the mean number of plant fragments provided. On the other hand, regardless of the plant genera, females that were infected with Plasmodium at the end of the reproductive season had significantly provided less plant fragments to the nest before incubation than non-infected females. However, we did not find differences between these two groups of females after the onset of incubation. Following the ‘Nest protection hypothesis’, if secondary plants exert a repellent role against potential vectors of blood parasites, the provisioning of more plant fragments before incubation could have prolonged the time period during which the plant repellent properties were active and, thus, would have reduced the female's chances of being infected. Indeed, blue tit females spend more time in the nest during the onset of the breeding season and are therefore potentially more vulnerable to blood-parasite vectors at early stages. We expected that the amount of plant fragments would also be relevant during incubation, but our results suggest that the early nest environment (i.e. during nest construction and egg laying) is determinant for the female's parasitic status. Moreover, the estimated time of detection of Plasmodium in birds’ blood is at least 5 days from infection (i.e. time to maturation of the first generation of metacryptozoites in P. relictum; Huijben et al., Reference Huijben, Schaftenaar, Wijsman, Paaijmans and Takken2007), making it less likely that the prevalence of Plasmodium is affected by plant provisioning during the nestling period. Analysing the female's parasite status at the beginning of the study would have allowed testing this possibility. Unfortunately, females are more prone to desert their nest when blood sampled before incubation and, thus, we only captured them once.
Additionally, following the ‘Drug hypothesis’, females would have benefited from the immune-stimulant properties of secondary plants, when dealing with Plasmodium. Although we expected that the amount of plant fragments would be associated with the prevalence of the 3 haemoparasites, it is possible that the immuno-stimulant properties of secondary plants have a more prominent role against infections by Plasmodium, or that their repellent role is more effective against their vectors (i.e. species of Culicidae family as Aedes, Anopheles and Culex; Valkiunas, Reference Valkiunas2004, LaPointe et al., Reference LaPointe, Goff and Atkinson2005, Martínez-de la Puente et al., Reference Martínez-de La Puente, Merino, Tomás, Moreno, Morales, Lobato, Talavera and Sarto I Monteys2009).
Nonetheless, when analysing the presence of particular plant genera, we found various significant associations with the 3 avian haemosporidian parasites (see below). In total, we identified 7 plant genera provided by blue tit females, all of them defined as aromatic herbs, and their occurrence being similar than that observed in other studies in the blue tit (Petit et al., Reference Petit, Hossaert-McKey, Perret, Blondel and Lambrechts2002). Interestingly, the presence of Achillea sp. herbs in the nests was negatively related to the female's blood-parasite infection, at least for Plasmodium and Haemoproteus. Females that had not provided Achillea sp. herbs before incubation were more likely to be infected by either Plasmodium or Haemoproteus than females with Achillea sp. fragments in their nest. Moreover, the relationship with Plasmodium infection remained significant during incubation, although it was relatively weaker. Achillea sp. are broadly known for their wide range of secondary metabolites (e.g. more than 50 volatile compounds identified from the essential oil of Achillea sp.; Al-Snafi, Reference Al-Snafi2013), which have been traditionally used for their insect repellent properties and their antimicrobial, antioxidant and cytotoxic effects (Ahmadi, Reference Ahmadi2011; Al-Snafi, Reference Al-Snafi2013; Pirmohammadi et al., Reference Pirmohammadi, Shayeghi, Vatandoost, Abaei, Mohammadi, Bagheri, Khoobdel, Bakhshi, Pirmohammadi and Tavassoli2016). The abundance of Achillea sp. in the study area is lower at the beginning of the reproductive season and peaks during June-July GBIF.org, 2023; https://www.gbif.org). Yet, its occurrence in blue tit nests remained stable and even decreased slightly during the nestling period (i.e. Achillea sp. before incubation: 14.9%; incubation: 17.9%; nestling period: 12.1%). This implies that gathering Achillea herbs during the early stages of the breeding season might be more difficult for breeding females, but might create a nest environment that helps them to cope with blood-parasite infections.
Surprisingly, females that had provided Lavandula sp. herbs during the nestling period were more likely to be infected by Leucocytozoon. Additionally, the presence of Teucrium before the incubation was also positively related with Leucocytozoon infection. However, the latter results should be taken with caution given the low number of females providing Teucrium to their nest. Considering that Leucocytozoon has the fastest meront-to-gametocyte development among bird haemosporidians (i.e. 10–12 days; Valkiunas and Iezhova, Reference Valkiunas and Iezhova2018), we may speculate that females infected by Leucocytozoon provided Lavandula sp. to their nest in order to repel vectors and thus reduce mother-to-offspring transmission (Ashford et al., Reference Ashford, Wylliej and Newton1990; Chakarov et al., Reference Chakarov, Linke, Boerner, Goesmann, Krüger and Hoffman2015). Indeed, prior research has shown vector-mediated parent-to-offspring transmission in common buzzards (Buteo buteo, Chakarov et al., Reference Chakarov, Linke, Boerner, Goesmann, Krüger and Hoffman2015). This could explain why females doubled the provisioning of Lavandula sp. in the nest during the nestling period. The main constituent of Lavandula sp. essential oil is the linalool, a terpene with anti-protist properties, including antimalarial effects (Silva et al., Reference Silva, Rezende, Emery, Gosmann and Gnoatto2015; Caputo et al., Reference Caputo, Reguilon, Mińarro, De Feo and Rodriguez-Arias2018; Gabriel et al., Reference Gabriel, Sussmann, Kimura, Rodriguez, Verdaguer, Leite and Katzin2018), and, indeed, it inhibits the development of parasitic protists such as Plasmodium, Haemoproteus and Leucocytozoon (Goulart et al., Reference Goulart, Kimura, Peres, Couto, Duarte and Katzin2004; Guggisberg et al., Reference Guggisberg, Amthor and Odom2014). Furthermore, Thymus sp. is also characterized by its linalool and thymol components (Rasooli and Mirmostafa, Reference Rasooli and Mirmostafa2003; Rota et al., Reference Rota, Herrera, Marínez, Sotomayor and Jordán2008; Boaventura et al., Reference Boaventura, dos Santos, de Sena Souza, Batista, Júlio and Luz2022), and was notably more abundant during the nestling period too. An additional possibility is that infected females provided aromatic herbs as a self-medication strategy against Leucocytozoon infection (Lozano, Reference Lozano, Møller, Milinski and Slater1998; De Roode et al., Reference De Roode, Lefev̀re and Hunter2013; Bravo et al., Reference Bravo, Bautista, García-París, Blanco and Alonso2014).
An alternative explanation for our results is that females infected by Plasmodium and Haemoproteus were in worse condition and, therefore, they were not able to seek specific plants or to provide as many plant fragments to their nest as uninfected females. This would support a trade-off between condition or immune status and the allocation of plant resources to the nest. Indeed, provisioning behaviour of secondary nest materials is highly demanding in terms of energy and time (Moreno et al., Reference Moreno, Soler, Møller and Linden1994). Nonetheless, female body mass, as a proxy of female condition, was not associated with blood-parasite status, which does not support the possible trade-off (Tables S2 and S3). Moreover, the higher provisioning of Lavandula and Teucrium by Leucocytozoon-infected females, perhaps as a self-medication strategy or as a way to reduce nestling infection, does not support this explanation either. Although we cannot totally discard the existence of a trade-off between condition and plant provisioning behaviour, we consider the repellent role of secondary plants against blood-parasite vectors as a stronger possibility. Assessing the relationship between immune responsiveness and aromatic plants would have helped to tease apart these possibilities. Further studies measuring immune responsiveness (e.g. by inducing immune activation to a novel antigen; e.g. as in Morales et al., Reference Morales, Moreno, Merino, Tomás, Martínez and Garamszegi2004, Reference Morales, Moreno, Lobato, Merino, Tomás, Martínez De La Puente and Martínez2006; Tomás et al., Reference Tomás, Merino, Moreno, Morales and Martínez-de La Puente2007) could shed light in in this question.
According to the ‘Nest protection hypothesis’, we predicted that secondary plant provisioning would prevent the development of nest-dwelling ectoparasites (i.e. blowfly pupae P. azurea and mites D. gallinoides). However, contrary to previous studies (reviewed in Scott-Baumann and Morgan, Reference Scott-Baumann and Morgan2015; Yang et al., Reference Yang, Ye, Huo, Møller, Liang and Feeney2020; Garrido-Bautista et al., Reference Garrido-Bautista, Ramos, Arce, Melero-Romero, Ferreira, Santos-Baena and Norte2023), the occurrence of mites in the nest was not associated with the mean number of fragments provided (but see Scott-Baumann et al., Reference Scott-Baumann, Morgan and Cogan2022), nor with the occurrence of any secondary plant genus. It is possible, however, that the presence of plant fragments reduces the level of infestation by ectoparasites (i.e. see Martínez-de la Puente et al., Reference Martínez-de La Puente, Merino, Tomás, Moreno, Morales, Lobato and Martínez2011; Tomás et al., Reference Tomás, Merino, Martínez-de la Puente, Moreno, Morales, Lobato and del Cerro2012), which we did not measure. If we had explored mite abundance/intensity rather than mite prevalence, perhaps we would have found a significant association. Interestingly, however, we did find a significant association with the number of blowflies, which was higher in nests with less plant fragments before incubation. These results partly support our initial prediction and are consistent with the ‘Nest protection hypothesis’. Moreover, when exploring particular plant genera and the presence/absence of blowflies, we found that nests containing Lamium sp. fragments before incubation were less likely to be infested by Protocalliphora, which may suggest that Lamium herbs create an adverse nest environment for the development of Protocalliphora larval stages (e.g. Lambrechts and Dos Santos, Reference Lambrechts and Dos Santos2000, Lafuma et al., Reference Lafuma, Lambrechts and Raymond2001; but see Mennerat et al., Reference Mennerat, Perret, Caro, Heeb and Lambrechts2008).
Finally, we did not find associations between the final plant dry mass (i.e. the dry mass of all the plant fragments extracted from the nest material after fledging) and the occurrence/abundance of blood parasites or ectoparasites. Blue tits differ from other bird species (e.g. starlings), as females incorporate fresh plant fragments while eliminating older ones (Lambrechts and Dos Santos, Reference Lambrechts and Dos Santos2000; Petit et al., Reference Petit, Hossaert-McKey, Perret, Blondel and Lambrechts2002). Thus, final dry mass represents the total mass of plants present during the nestling period, which might not be necessarily the same at previous stages of reproduction. It is possible, however, that plant provisioning during the nestling period does affect the immune status of the nestlings and not of the females. Therefore, additional investigations on offspring health will be necessary to understand plant provisioning behaviour at the end of reproduction.
Conclusions
Our results show that the provisioning of specific secondary plants by blue tit females was in part associated with their infection status by haemosporidian parasites (i.e. Plasmodium, Haemoproteus, Leucocytozoon), but that such associations changed throughout the breeding season. In particular, females that had provided fewer plant fragments before incubation were more likely to be infected by Plasmodium. Moreover, females that had provided Achillea sp. fragments at early stages of reproduction were less likely to be infected by both Plasmodium and Haemoproteus. Thus, secondary plants may create an early nest environment that may repel the blood-parasite vectors (‘Nest protection hypothesis’) or directly favour that females cope with infection (‘Drug hypothesis’). However, contrary to our predictions, Leucocytozoon infection was positively related to the presence of other plant species (Teucrium sp. and Lavandula sp.). Due to the frequent parent-offspring transmission of Leucozytozoon in avian nests, but not of the other 2 haemoparasites, it is possible that females already infected by Leucozytozoon increased plant provisioning to prevent such transmission or as a self-medication strategy. Our study provided indirect evidence both for the ‘Nest protection hypothesis’ and the ‘Drug hypothesis’, as we did not measure vector numbers in nests (Tomás et al., Reference Tomás, Merino, Martínez-de La Puente, Moreno, Morales and Lobato2008) nor the immune system or other physiological responses of blue tit females (Morales et al., Reference Morales, Moreno, Merino, Tomás, Martínez and Garamszegi2004). Due to the correlative nature of this study, we cannot discern the causal role of the associations that we found. However, to our knowledge, this study constitutes the first approximation to exploring the link between plant provisioning behaviour and the blood-parasite status of the females, which are the plant gatherers in this bird species. We hope that this study serves as a starting point for future experimental research in this topic.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182024000775.
Data availability statement
García-Campa, Jorge; González-Braojos, Sonia; Morales, Judith (2024). Nest secondary plants and their associations with haemosporidian blood parasites in blue tit females. figshare. Dataset. https://doi.org/10.6084/m9.figshare.26348296.v1
Acknowledgements
We thank Daniel Díaz and Alejandra Mira (from the Centro de Gestión del Parque Regional de la Cuenca Alta del Río Manzanares) for logistic support. We appreciate the constructive feedback and insightful comments provided by the editor, the anonymous reviewer, and Jorge Garrido-Bautista. Their comments have substantially improved the final version of this manuscript. We thank the support for the publication fee by the CSIC Open Access Publication Support Initiative through its Unit of Information Resources for research (URICI).
Authors’ contributions
J.G-C. and S.G-B. contributed equally to this work. J.G-C., S.G-B. and J.M. conceived and designed the study, collected field data and performed the statistical analyses. S.G-B. analysed the blood parasites, nest ectoparasites and revised the identification of plant genera. J.G-C. wrote the manuscript with input from all authors. All authors read, reviewed and approved the final manuscript.
Financial support
Projects PID2019-106032GB-I00, PID2022-139166NB-I00 and 2023AEP111 (to JM) funded by MCIN/AEI/https://doi.org/10.13039/501100011033 and by ‘ERDF A way of making Europe’. JG-C was supported by Grant BES-2017-079750 funded by MCIN.
Competing interests
None.
Ethical standards
All the methods were performed in accordance with the Spanish laws in relation to animal research. The study licenses to perform the experimental protocols were approved by the Spanish Research Council (CSIC, ref. 639/2017) and the Consejería de Medio Ambiente, Administración Local y Ordenación del Territorio, Comunidad de Madrid (ref. 10/056536.9/18; PROEX 237/17). The study was conducted in compliance with the ARRIVE guidelines.