Article contents
Molecular typing of Strongyloides stercoralis in Latin America, the clinical connection
Published online by Cambridge University Press: 06 September 2021
Abstract
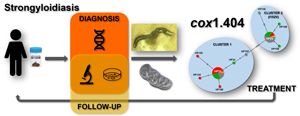
This study analysed Strongyloides stercoralis genetic variability based on a 404 bp region of the cox1 gene from Latin-American samples in a clinical context including epidemiological, diagnosis and follow-up variables. A prospective, descriptive, observational study was conducted to evaluate clinical and parasitological evolution after ivermectin treatment of 41 patients infected with S. stercoralis. Reactivation of the disease was defined both by clinical symptoms appearance and/or direct larvae detection 30 days after treatment or later. We described 10 haplotypes organized in two clusters. Most frequent variants were also described in the Asian continent in human (HP24 and HP93) and canine (HP24) samples. Clinical presentation (intestinal, severe, cutaneous and asymptomatic), immunological status and eosinophil count were not associated with specific haplotypes or clusters. Nevertheless, presence of cluster 1 haplotypes during diagnosis increased the risk of reactivation with an odds ratio (OR) of 7.51 [confidence interval (CI) 95% 1.38–44.29, P = 0.026]. In contrast, reactivation probability was 83 times lower if cluster 2 (I152V mutation) was detected (OR = 0.17, CI 95% 0.02–0.80, P = 0.02). This is the first analysis of S. stercoralis cox1 diversity in the clinical context. Determination of clusters during the diagnosis could facilitate and improve the design of follow-up strategies to prevent severe reactivations of this chronic disease.
Keywords
- Type
- Research Article
- Information
- Copyright
- Copyright © The Author(s), 2021. Published by Cambridge University Press
Footnotes
These authors contributed equally to this work.
References
- 6
- Cited by



