Introduction
Avian colonial breeding is often associated with higher parasite prevalence and intensity, as the increased proximity and contact between different group members facilitate ectoparasite transmission (Brown and Brown, Reference Brown and Brown1986; Côté and Poulin, Reference Côté and Poulin1995; Tella, Reference Tella2002; Patterson and Ruckstuhl, Reference Patterson and Ruckstuhl2013). This pattern differs according to the mode of ectoparasite transmission, with contact-transmitted parasites (e.g. mites or lice) being positively correlated with group size, whereas no correlation is expected for mobile parasites (e.g. biting flies and mosquitoes) due to encounter dilution effects (Patterson and Ruckstuhl, Reference Patterson and Ruckstuhl2013, but see Veiga et al., Reference Veiga, Václav and Valera2020). Parasites can directly or indirectly affect the reproductive and survival components of individual fitness by decreasing nestling body condition and survival, or by reducing adult fecundity (Brown and Brown, Reference Brown and Brown1986; Merino and Potti, Reference Merino and Potti1995; Arriero and Møller, Reference Arriero and Møller2008; Hoi et al., Reference Hoi, Darolová, Krištofík and Hoi2018). This may consequently impact avian population dynamics, for example by decreasing breeding-site fidelity and foster both the formation and extinction of colonies (Brown and Brown, Reference Brown and Brown2004; Calabuig et al., Reference Calabuig, Ortego, Cordero and Aparicio2010; Brown et al., Reference Brown, Roche and Brown2017; Sanz-Aguilar et al., Reference Sanz-Aguilar, Payo-Payo, Rotger, Yousfi, Moutailler, Beck, Dumarest, Igual, Miranda, Torres, Picorelli, Gamble and Boulinier2020). Group living may also result in increased physiological social stress due to increased competition for resources, which synergizes with host susceptibility to parasite infestation (Beldomenico and Begon, Reference Beldomenico and Begon2010; Kappeler et al., Reference Kappeler, Cremer and Nunn2015). However, most studies assessing the relationship between parasitism and colonial breeding targeted monospecific colonies or the interaction between a single host and a single parasite species, overlooking the likely interactions among different hosts, or different parasites within the same host – the parasite infracommunity (Bush et al., Reference Bush, Lafferty, Lotz and Shostak1997; Holt et al., Reference Holt, Dobson, Begon, Bowers and Schauber2003; Poulin, Reference Poulin2007; but see Whiteman and Parker, Reference Whiteman and Parker2004; Veiga and Valera, Reference Veiga and Valera2020). In fact, bird colonies are often composed of different species, resulting in mixed-species colonies, that reshape the structure and functioning of communities through the increased interaction between the grouping species (Gaglio et al., Reference Gaglio, Sherley, Cook, Ryan and Flower2018; Catry and Catry, Reference Catry and Catry2019). The formation of mixed-species colonies may allow species to acquire the benefits of group living while reducing the costs associated with intraspecific competition (Møller et al., Reference Møller, Dufva and Allander1993; Campobello et al., Reference Campobello, Sarà and Hare2012), although multi-species associations may also result in costs through factors such as kleptoparasitism or interspecific competition (Gaglio et al., Reference Gaglio, Sherley, Cook, Ryan and Flower2018; Catry and Catry, Reference Catry and Catry2019).
In multi-species assemblages, intra- and interspecific social contacts likely increase and complexify the interactions between different host and parasite species (Valera et al., Reference Valera, Casas-Crivillé and Hoi2003; Keesing et al., Reference Keesing, Holt and Ostfeld2006). High host diversity could decrease infestation risk through dilution effects, as different host species may be differently susceptible and/or competent to different parasite species, but this effect may not always occur and likely depends on specific community compositions (Keesing et al., Reference Keesing, Holt and Ostfeld2006; Randolph and Dobson, Reference Randolph and Dobson2012; Civitello et al., Reference Civitello, Choen, Fatima, Halstead, Liriano, McMahon, Ortega, Sauer, Sehgal, Young and Rohr2015; Halsey, Reference Halsey2018; Martin et al., Reference Martin, Addison, Bean, Buchanan, Crino, Eastwood, Flies, Hamede, Hill, Klaassen, Koch, Martens, Napolitano, Narayan, Peacock, Peel, Peters, Raven, Risely, Roast, Rollins, Ruiz-Aravena, Selechnik, Stokes, Ujvari and Grogan2019). Mixed-species assemblages may also promote interspecific parasite exchange, especially within generalist parasite species. Whether the occurrence of several host species in bird assemblages increases or decreases the prevalence and abundance of parasites and their relationship with colony size or density remains poorly studied (Tella et al., Reference Tella, Gajón, Gortázar and Osácar1998; Valera et al., Reference Valera, Casas-Crivillé and Hoi2003; Veiga et al., Reference Veiga, Václav and Valera2020).
Study of the ecological relationship between parasites in mixed-species colonies may be harder than in mono-specific groups due to the complexity of addressing several species and their interactions, with the solution often being to simplify the system into one–one actors. In this study, we investigate host–parasite relationships in mixed-species colonies, by considering several host and parasite species and by simultaneously looking at several colony traits that may influence the prevalence and abundance of ectoparasites. In southwestern Iberia, colonial lesser kestrels Falco naumanni breed in man-made structures that attract other species, including common kestrels Falco tinnunculus, European rollers Coracias garrulus, barn owls Tyto alba, little owls Athene noctua, jackdaws Corvus monedula, spotless starlings Sturnus unicolor and feral pigeons Columba livia; thus forming mixed-species colonies (Catry and Catry, Reference Catry and Catry2019). These multi-species assemblages provide an ideal opportunity to understand the ecological relationships between multiple host and parasite species (Valera et al., Reference Valera, Casas-Crivillé and Hoi2003). Here, we study how colony traits such as colony size, host species richness, density, and composition, influence the ectoparasite infracommunities. Specifically, we aim to: (1) describe the ectoparasite infracommunity of different avian host species breeding in mixed-species colonies; (2) determine the main colony traits potentially affecting ectoparasite infracommunity composition and (3) assess how colony traits drive the abundance of Carnus hemapterus, a widespread haematophagous fly parasitizing at least 64 host species from 24 avian families (Grimaldi, Reference Grimaldi1997; Brake, Reference Brake2011) and the most common ectoparasite in our study area. For this, we study the ectoparasites in nestlings and nests of the four main common bird species in 30 mixed-species colonies.
Materials and methods
Study system: hosts and parasites
This study took place in the Castro Verde Special Protection Area, Southern Portugal (~37°43′N, 7°57′W) (Fig. 1). In the framework of European LIFE projects to recover lesser kestrel populations, more than 800 artificial nests were provided in the study area, including new cavities opened in abandoned rural buildings, artificial cavities in newly built breeding walls and towers with up to 90 cavities, wooden nest-boxes and clay pots (Catry et al., Reference Catry, Alcazar, Franco and Sutherland2009). Given the low availability of nest-sites in the area, nest-site provisioning was very effective at increasing the lesser kestrel population and attracted other bird species to nest in these structures (Catry et al., Reference Catry, Alcazar, Franco and Sutherland2009; Catry and Catry, Reference Catry and Catry2019; Gameiro et al., Reference Gameiro, Franco, Catry, Palmeirim and Catry2020).

Fig. 1. Location of small (white), medium (light) and large (dark) colonies sampled in this study (See online for the colour version). Boundaries of the Castro Verde Special Protection Area (SPA) shown as a black line.
Mixed-species colonies occur in adobe-built abandoned farmhouses (nests located in cavities that appear with building degradation – ‘natural cavities’) or in artificial nest structures. Here, ‘colony’ was defined as a site with at least two active nests – usually a single building, or a main building with few smaller, annex structures (e.g. a farmhouse with a separated shed). Because nests occur inside cavities, the maximum size of the colony is ultimately dependent on the number of available nest-sites, which can go from just a couple to more than a hundred cavities. Larger colonies (>40 breeding pairs) tend to have higher density of nests and a higher richness of host species and can be dominated by either lesser kestrels or spotless starlings (Table 1). Larger colonies usually also hold a few pairs of jackdaws and feral pigeons, one to four common kestrels and European rollers and one pair of barn owl and/or little owl (Catry and Catry, Reference Catry and Catry2019). Distance to nearest colony was always greater than 100 m (min = 150 m, max = 4266 m).
Table 1. Colony traits of small (<20 pairs), medium (21–40 pairs) and large (>40 pairs) mixed-species colonies sampled for ectoparasites

Median (min–max) values are shown for host richness, colony size, density (average distance to the three active closest nests) and colony composition (number of active nests).
a Also includes other, less abundant host species (common kestrels, jackdaws, little owls and barn owls).
This study focused on the four most common bird species in these mixed-species colonies: lesser kestrels, European rollers (hereafter rollers), spotless starlings (hereafter starlings) and feral pigeons (hereafter pigeons). Lesser kestrels and rollers are single-brooded, secondary cavity nesting birds with a trans-Saharan migration (Catry et al., Reference Catry, Catry, Patto, Franco and Moreira2015). Lesser kestrels are typically colonial, with colonies reaching up to 80 breeding pairs in the study area (Catry et al., Reference Catry, Alcazar, Franco and Sutherland2009). They arrive at the breeding grounds in early February and typically lay four to five eggs in April–May. Nestlings are born with a down feather coat after a 28 days incubation and usually fledge at 36 days (Catry et al., Reference Catry, Catry, Patto, Franco and Moreira2015). Rollers (up to four breeding pairs) are usually found breeding in lesser kestrel colonies but can also nest in isolated nests in farmhouses or in nest-boxes placed on trees or telephone poles. Egg laying is asynchronous with three to six eggs laid in May–June. Nestlings are born featherless after 17–19 days of incubation and fledge at 20–25 days (Catry et al., Reference Catry, Catry, Patto, Franco and Moreira2015).
Starlings and pigeons are resident in the study area and lay several clutches along the breeding season. Starlings generally nest at high densities, with up to 32 breeding pairs in these mixed-species colonies (Table 1). Starlings lay three to six eggs, incubation takes ca. 12 days and nestlings fledge at 20–22 days old (Muriel et al., Reference Muriel, Pérez-Rodríguez, Puerta and Gil2013). Pigeons occur at lower densities, with up to eight breeding pairs in these colonies (Table 1). They lay up to two eggs, incubation lasts around 18 days and nestlings fledge at ca. 28 days old (Johnston and Janiga, Reference Johnston and Janiga1995). Contrary to lesser kestrel and roller nests, which are usually just comprised of dirt and prey remains, starlings and pigeons build their nest with sticks, straws and other vegetable material, and pigeons also accumulate droppings inside the cavity.
The most common ectoparasites found in hosts' nestlings in our study area were carnid flies (C. hemapterus, Diptera: Carnidae), haematophagous mites (Acari: Mesostigmata), louse flies (Diptera: Hippoboscidae) and feather lice (Phthiraptera); and they were considered in this study as four separate groups since: (i) identification at the species level and quantification of each species in each nest and host was not feasible, and (ii) working at the species level falls beyond the scope of this study, i.e. to investigate the influence of colony traits on the general patterns of ectoparasite infestation. Yet, some information about the identified species is provided in the ‘Discussion’ section.
The generalist C. hemapterus (hereafter Carnus) is a nidicolous, haematophagous fly infesting many bird taxa. Carnus loses its wings after finding a host, with peak infestations occurring during the mid-nestling stage, and with a resistance stage (pupa) that remains in the nest debris until the next breeding season when adult flies emerge (Valera et al., Reference Valera, Casas-Crivillé and Calero-Torralbo2006a; Calero-Torralbo and Valera, Reference Calero-Torralbo and Valera2008). Despite being a mobile ectoparasite, it was found to increase with colony size and host density (Hoi et al., Reference Hoi, Krištofík, Darolová and Hoi2010; Veiga et al., Reference Veiga, Václav and Valera2020; but see Liker et al., Reference Liker, Márkus, Vozár, Zemankovics and Rózsa2001). Mites are also generalist ectoparasites, feeding mostly on blood or skin tissue of nestlings and adults. Mite populations usually grow quickly in the nest during their host's breeding season and some individuals may overwinter in the nest (Burtt et al., Reference Burtt, Chow, Babbit, Loye and Zuk1991). Being contact transmitted parasites, they are expected to increase with colony size (Davis and Brown, Reference Davis and Brown1999). Louse flies are also haematophagous parasites, but imagoes do not lose their wings and can fly between nests, and they have often a more restricted host range (Veiga et al., Reference Veiga, De Oña, Salazar and Valera2019). Imagoes spend most of their time feeding on the host's body, and pupae are formed and apparently overwinter in the nest (Boyd, Reference Boyd1951). Louse flies are mobile parasites and should not be affected by host coloniality (Poulin, Reference Poulin1991). Feather chewing lice are permanent ectoparasites with a host range usually confined to species within the same family or genus (Clayton et al., Reference Clayton, Adams, Bush, Atkinson, Thomas and Hunter2008). They live on the feathers and seldom leave their host except to transfer among individuals through direct contact (e.g. between parents and their offspring), and thus are likely influenced by host sociality (Clayton and Tompkins, Reference Clayton and Tompkins1995; Rózsa et al., Reference Rózsa, Rékási and Reiczigel1996; Whiteman and Parker, Reference Whiteman and Parker2004; Ortego et al., Reference Ortego, Aparicio, Calabuig and Cordero2007).
Data collection
Ectoparasite estimation
Fieldwork was conducted during the breeding season of 2018, from mid-April to mid-July, by sampling unclean nests occupied by the four studied host species. Colonies and nests within colonies were randomly sampled during a lesser kestrel and roller monitoring programme. A total of 30 colonies were sampled (Fig. 1): seven with all four hosts; four with lesser kestrels, rollers and starlings; two with lesser kestrels, rollers and pigeons; six with lesser kestrels and rollers; two with lesser kestrels and pigeons; two with rollers and pigeons; five with lesser kestrels and two with rollers (Table 1). Overall, we sampled 261 nests: 141 lesser kestrel, 33 roller, 38 pigeon and 49 starling nests. Nests were not cleaned prior to this study for two reasons: (i) removing old nest detritus was not possible for most of these nests (especially for natural cavities) and (ii) unclean nests provide results that better resemble the natural conditions (Møller, Reference Møller1989). Soiled nests may influence the prevalence and numbers of some ectoparasites in these colonies and may cause some unaccounted variability in the data, particularly when considering ectoparasites with most of their life cycle, including long diapauses, occurring in the nests (Veiga et al., Reference Veiga, Václav and Valera2020). Thus, this potential bias was considered in the statistical approach (see ‘Data analysis’ section) and its influence was discussed.
The presence and number of ectoparasites were assessed by examining all nestlings in each host species nest. Each nest was sampled at two different periods: at mid-nestling stage (around 8–12 days old), and few days before fledging. During each of these two periods, each nestling was taken from its nest and placed in a transparent plastic bag to avoid losing mobile parasites (e.g. louse flies). The nestling was then taken from the bag and the number of carnid flies and louse flies on the bag and on the body surface and sheaths of the nestling were counted twice and then averaged (Roulin, Reference Roulin1998; Václav et al., Reference Václav, Calero-Torralbo and Valera2008). Feather lice were sampled by carefully scanning the nestlings' sheaths and feathers (Valera et al., Reference Valera, Casas-Crivillé and Hoi2003; Ortego et al., Reference Ortego, Aparicio, Calabuig and Cordero2007). Mites were sampled by resting the observer's hand on the bottom of the nest for 1 min and then the number of mites were counted twice and then averaged. Nestlings were then carefully placed back on the nests. The number of each ectoparasite group was calculated as the sum of parasites in all nestlings from the same brood. The maximum number of parasites from both sampling periods was selected. All study protocols were approved by the relevant Portuguese authorities (Instituto da Conservação da Natureza e das Florestas).
Mixed-species colony traits
The following variables were recorded for each sampled nest: host species identity, type of nest (natural cavities in farmhouses, artificial cavities, clay pots and wooden nest-boxes), brood size (number of nestlings) and the sampling date of the first sampling period, which was used as a proxy for ectoparasite seasonal effects (Calero-Torralbo et al., Reference Calero-Torralbo, Václav and Valera2013). The species occupying the focal nest in the previous year was also recorded, as some ectoparasites spend most of their life cycle in the nest and their occurrence and abundance may depend on previous breeding seasons (Valera et al., Reference Valera, Casas-Crivillé and Calero-Torralbo2006a). Each nest was also categorized according to four main colony traits: colony host richness (number of host species in the colony); colony size (number of active nests, i.e. nests with nestlings, of all species); colony density (the inverse of the average distance to the three active closest nests, in meters) and colony composition (four variables, each with the number of active nests of each of the four main host species) (Table 1). Colony size was grouped into three categories: small (up to 20 nests), medium (21–40 nests) and large (more than 40 nests) (Fig. 1). Similarly, colony density was grouped into low (average distance to the three closest nests >5 m), medium (1–5 m) and high density (<1 m). All traits (except for colony composition) acknowledged all nesting bird species, including less abundant species (common kestrels, jackdaws, little owls and barn owls).
Data analysis
The nest was used as the sampling unit and thus the term infracommunity refers to the community of ectoparasites in a given nest (see Veiga and Valera, Reference Veiga and Valera2020 for a similar approach). Accordingly, the prevalence and mean intensity of each ectoparasite group for each host species was calculated, respectively, as the proportion of infested nests among all nests sampled and the mean number of parasites of all infested nests. Because some nests were not sampled during both periods, prevalence and mean intensity of each parasite were calculated only for nests sampled at each parasite's peak infestation stage (assessed from our own dataset and the literature; see following sentences), so that comparisons could be made between the different hosts (see ‘Results’ section). Carnus infestation peaks at mid-nestling stage (prevalence of 87% and 20% for the first and second sampling periods, respectively; see also Václav et al., Reference Václav, Calero-Torralbo and Valera2008), while feather lice and louse flies are more common in fully-grown, feathered nestlings (lice prevalence of 2% and 86%, and louse fly prevalence of 26% and 47% for the first and the second sampling periods, respectively; see also Muñoz et al., Reference Muñoz, Pomarol, Castella, Gutierrez and Galmes1993). The prevalence of mites did not differ between the two sampling periods in our study (23% and 19% for the first and second sampling periods, respectively). Accordingly, prevalence and mean intensity values of carnid flies were obtained from all nests sampled in the first period, values for lice and louse flies from nests sampled in the second period and values for mites were obtained from all nests (sampled both in the first and/or in the second period).
Fisher's exact tests, and Kruskal–Wallis H test followed by bootstrap two-sample t-tests, were used for comparing parasite prevalence and mean intensity, respectively, among hosts, using 2000 replications for both estimation of confidence intervals and bootstrap t-tests (Rózsa et al., Reference Rózsa, Reiczigel and Majoros2000; Veiga et al., Reference Veiga, De Oña, Salazar and Valera2019).
A principal component analysis was used to explore the potential correlation among the colony variables without constraints (random factors), prior to further analysis (Supplementary Fig. S1) (Borcard et al., Reference Borcard, Gillet and Legendre2011; Václav and Valera, Reference Václav and Valera2018). To examine how colony traits influence the ectoparasite infracommunity composition, a partial canonical correspondence analysis (CCA) was used including all sampled nests and conditioned by brood size, sampling date and colony ID (Borcard et al., Reference Borcard, Gillet and Legendre2011). The presence–absence matrix of each parasite group in each nest was used as the response variable (nests without parasites were removed; Oksanen, Reference Oksanen2020). Host species, nest type, the species using the nest in the previous year, and colony host richness, size, density, and composition, were used as the predictive variables. The variance inflation factor (VIF) was used to assess collinearity between predictive variables (all VIFs <10, min = 1.01, max = 6.61) and forward and backward selections were performed to choose the best CCA model (Borcard et al., Reference Borcard, Gillet and Legendre2011; Václav and Valera, Reference Václav and Valera2018).
To assess how colony traits (richness, size, density, and composition) influence the abundance (i.e. including both infested and non-infested nests) of Carnus, the most common ectoparasite in our mixed-species colonies, a generalized linear mixed model (GLMM) was used. The model followed a negative binomial distribution of errors to account for the aggregated distribution of Carnus among hosts (Václav and Valera, Reference Václav and Valera2018). Carnus abundance in each nest was used as the response variable and colony ID as a random variable. The sample-to-variable ratio and the high correlation between some colony traits did now allow the inclusion of all variables in a single model (Supplementary Fig. S1). Because colony size, density and host richness were correlated, the number of variables were reduced by choosing the most meaningful traits. Colony size was removed in favour of colony density because the latter reports about the nest density around each focal nest, whereas colony size attributes the same value to all nests within the same colony. Colony composition was selected instead of colony host richness because it contains information about the identity of the host and its abundance, rather than just the number of species found in a given colony. However, because the abundance of pigeons and rollers was considerably lower than the one of lesser kestrels and starlings, and both were correlated with the number of starling nests (Supplementary Fig. S1), colony composition was restricted to the number of nests of the most abundant hosts, i.e. lesser kestrels and starlings. Nonetheless, different combinations of variables were tested, which also included the host species using the nest in the previous year, but did not provide additional or different outputs. The results shown here refer to the most biologically meaningful model, that includes the following, non-correlated, explanatory variables: host species, brood size, sampling date, type of nest, colony density (categorical), number of lesser kestrel nests and number of starling nests (highest VIF = 5.07, for type of nest). A model-averaging approach based on the Akaike information criterion (AIC) was used to obtain weighted average estimates for fixed parameters (Grueber et al., Reference Grueber, Nakagawa, Laws and Jamieson2011). Model averaging was performed on models with the cumulative sum of corrected AIC (AICc) weights >0.95.
All continuous explanatory variables were scaled and centred prior to analysis. All analyses were conducted with R software 3.6.1 (R Core Team, 2016), using the packages Vegan 2.5-6 (Oksanen, Reference Oksanen2020), nlme 3.1-148 (Pinheiro et al., Reference Pinheiro, Bates, DebRoy and Sarkar2020), lme4 1.1-23 (Bates et al., Reference Bates, Maechler, Bolker, Walker, Christensen, Singmann, Dai, Scheipl, Groethendieck, Green, Fox, Bauer and Krivitsky2020) and MuMIn 1.43.17 (Bartón, Reference Bartón2020).
Results
Prevalence and intensity of ectoparasites in mixed-species colonies
The composition of the infracommunity of ectoparasites differed among the various host species breeding in mixed-species colonies: lesser kestrels and pigeons had all four types of ectoparasites whereas starlings had no louse flies and rollers had no lice and almost no louse flies (Table 2).
Table 2. Prevalence (percentage of infested nests) and mean intensity (mean number of parasites found in infested nests) of ectoparasites found on nests of mixed-species colonies in southern Portugal (with 95% confidence intervals in round brackets and number of infested nests/number of sampled nests in square brackets).

Carnus was the most common ectoparasite in mixed-species colonies but its prevalence differed among the four host species (Fisher test: P < 0.001), being highest in lesser kestrels and rollers and lowest in pigeons. Carnus mean intensity was more than double in rollers than in lesser kestrels (bootstrap two-sample t-test: t = 4.22; d.f. = 29.65, P < 0.001) and lowest in pigeons (Table 2). Mites were the second most common ectoparasite in mixed-species colonies, reaching the lowest prevalence and intensity in lesser kestrels (Table 2). Rollers, pigeons and starlings had similar mite prevalence and intensity (prevalence; Fisher test: P = 0.97 intensity: Kruskal–Wallis H: 1.11, d.f. = 2, P = 0.57). Lice were almost exclusively found in lesser kestrels and pigeons, with similar prevalence and intensity (prevalence: Fisher test: P = 1.00; intensity: bootstrap two-sample t-test: t = −1.45, d.f. = 22.65, P = 0.160) and louse flies were mostly found in pigeon nests (Table 2).
Influence of colony traits on ectoparasite infracommunity in mixed-species colonies
The partial CCA, conditioned by sampling date, brood size and colony ID, revealed that host species was the only predictor of parasite infracommunity (presence/absence), being the only variable selected following both forward and backward selection processes (final CCA adjusted R 2 = 0.18; χ 2 = 0.2, F = 18.2, P value = 0.001). Conditioned and constrained partitioning explained 27.0% and 16.7% of the total inertia, respectively (‘variance’, total inertial = 1.11). The ectoparasite infracommunity of each host species was best described by the presence of louse flies, followed by lice and mites (Fig. 2). Louse flies were clearly associated with pigeons and lice commonly infested pigeons and lesser kestrels. Mites showed a positive association with starlings, rollers and pigeons, whereas Carnus was negatively associated with pigeons (see also Table 2).

Fig. 2. Biplot (scaling 3) of the partial CCA showing the relationship between the occurrence of ectoparasites and host species, the only explanatory variable selected after forward and backward selections. Statistics: CCA1: χ 2 = 0.10, F = 29.0, P value = 0.001; CCA2: χ 2 = 0.08, F = 24.9, P value = 0.001. Sample size = 240 nests (139 lesser kestrels, 29 rollers, 33 pigeons, 39 starlings).
Influence of colony traits on C. haemapterus abundance in mixed-species colonies
Host species had a significant influence on Carnus abundance, being highest in rollers and followed by lesser kestrels, starlings and pigeons (the reference level) (Table 3). Model averaging revealed that Carnus abundance increased significantly with brood size and marginally significantly with increasing numbers of lesser kestrel nests in the colony. In contrast, it decreased significantly with increasing numbers of starlings' nests. The abundance of carnid flies also decreased, although marginally significantly, in clay pots when compared to other types of nests. Colony density had no influence on Carnus abundance. The models with ΔAICc <2 after model averaging and the best two models can be found in Tables S1 and S2, respectively, in the Supplementary material.
Table 3. Results of model averaging on the effect of colony traits on Carnus hemapterus abundance in mixed-species colonies using nests from all four host species (lesser kestrels, rollers, pigeons, and starlings). Variables with a significant effect (P value < 0.05) are given in bold.

Entry model: Carnus abundance ~ host species + brood size + sampling date + nest type + colony density (Cat) + number of lesser kestrel nests + number of starling nests + (1|colony ID); sample size = 230 nests (132 lesser kestrels, 28 rollers, 31 pigeons, 39 starlings).
Values reported are conditional averages and adjusted s.e. Carnus abundance was studied using GLMMs following a negative binomial distribution of errors to account for its aggregated distribution among hosts. Colony ID was used as random factors. N, number of; Cat, categorical; ID, identity.
Discussion
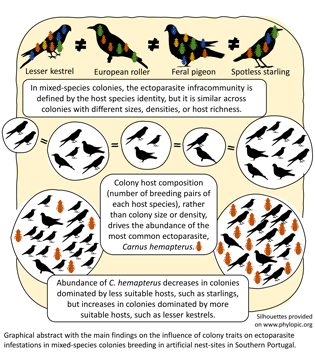
This study provides information on the ecology of avian parasitism in mixed-species colonies. To our knowledge, there is little information on the factors influencing host infestation by ectoparasites in colonies formed by multiple species of hosts and even less at the parasite infracommunity level. Four main ectoparasite groups – carnid flies, louse flies, lice, and mites – in four different host species were analysed, including the conservation reliant lesser kestrel and European roller (Gameiro et al., Reference Gameiro, Franco, Catry, Palmeirim and Catry2020). It was found that the main driver for the differences in composition of the ectoparasite infracommunity was the identity of the host species, regardless of colony traits such as size, density or richness. Accordingly, variation in the abundance of the most common ectoparasite (Carnus) in these mixed-species assemblages was explained by colony composition (number of nests of each host species), rather than by overall colony size or density.
Ectoparasite infracommunity in birds breeding in mixed-species colonies
The importance of host species identity for ectoparasite ecology has already been described in small mammals (Krasnov et al., Reference Krasnov, Korallo-Vinarskaya, Vinarksi, Shenbrot, Mouillot and Poulin2008; Lareschi and Krasnov, Reference Lareschi and Krasnov2010). These results suggest that the four host species vary in their susceptibility (sensu Martin et al., Reference Martin, Addison, Bean, Buchanan, Crino, Eastwood, Flies, Hamede, Hill, Klaassen, Koch, Martens, Napolitano, Narayan, Peacock, Peel, Peters, Raven, Risely, Roast, Rollins, Ruiz-Aravena, Selechnik, Stokes, Ujvari and Grogan2019) to different ectoparasite groups (Keesing et al., Reference Keesing, Holt and Ostfeld2006). Even the prevalence and intensity of Carnus, the most common and broadly considered generalist species (Veiga et al., Reference Veiga, De Oña, Salazar and Valera2019), varied considerably between hosts. Nest- and host-related characteristics may account for some of the differences found, as suggested by our results on Carnus. Pigeons and starlings were the least attractive hosts. These bird species use vegetable material when building their nests, which was found to be avoided by Carnus (Valera et al., Reference Valera, Martín-Vivaldi and Carles-Tolrá2006b, Reference Valera, Veiga, Sandoval and Moreno2018). Pigeons also accumulate droppings inside the cavity that may limit the suitability of the detritus for the larval and pupal stages of this ectoparasite (Veiga et al., Reference Veiga, De Oña, Salazar and Valera2019). Carnid flies seemingly also prefer rollers to lesser kestrels since the former bird species holds more than twice as many Carnus as lesser kestrels. Nestling kestrels are born with a down feather coat that may hinder the access to blood by ectoparasites, when compared to the naked body of roller nestlings.
Mites also infested all host species but were less prevalent and abundant on lesser kestrels. In a dry climate like the one in the study area, the humidity required by mites (Nordenfors et al., Reference Nordenfors, Höglund and Uggla1999) may be secured by the vegetable material used in starling or pigeon nests, when compared to a mostly sandy substratum of lesser kestrel or roller nests. However, as rollers arrive later to the breeding grounds from their spring migration, they are likely to re-use a nest previously occupied by starlings or pigeons (hence more humid), potentially explaining why rollers had higher prevalence of mites compared to lesser kestrels (see below and Veiga et al., Reference Veiga, Václav and Valera2020).
The factors accounting for differences in the prevalence of lice and louse flies among host species are not so clear and their identification goes beyond the aims of our study. Nonetheless, it is worth reporting such differences in mixed colonies where the contact among species and individuals can facilitate transmission. Lice were mostly present on lesser kestrels and pigeons, with just a single louse found on one starling nestling and none on rollers. Although lice were not identified at the species level, these ectoparasites are considered species-specific and no studies were found reporting the same lice species infecting both pigeons and lesser kestrels (Rózsa, Reference Rózsa1990; Ortego et al., Reference Ortego, Aparicio, Calabuig and Cordero2007; Calabuig et al., Reference Calabuig, Ortego, Cordero and Aparicio2010; Galloway and Lamb, Reference Galloway and Lamb2015). Factors such as gregariousness can explain why species such as pigeons or lesser kestrels have many lice. In contrast, species usually breeding in low densities (e.g. rollers) could be less appropriate for lice. Because chewing lice are mostly found on feathers, they may be more common and abundant on post-fledging juveniles or adults, when compared to young nestlings (Liker et al., Reference Liker, Márkus, Vozár, Zemankovics and Rózsa2001; Ortego et al., Reference Ortego, Aparicio, Calabuig and Cordero2007). Regarding louse flies, these were found almost exclusively on pigeons, with only three lesser kestrel and one roller nest infested and no starling nest infested. Examination of six louse flies demonstrates that at least three different species occur in our study area: Pseudolynchia canariensis in pigeons and lesser kestrels, Ornithophila gestroi in lesser kestrels and Hippobosca longipennis in rollers. Pseudolynchia canariensis has a wide host range and is common in pigeons and in the Falco genus (Maa, Reference Maa1966), although to the authors' knowledge this the first time P. canariensis has been reported in lesser kestrels. Ornithophila gestroi has already been reported in lesser kestrels, with similar abundances but at higher prevalence than the one found in this study (Tella et al., Reference Tella, Forero, Donázar, Negro and Hiraldo1997). Hippobosca longipennis is an ectoparasite typically found in carnivores (Maa, Reference Maa1969; Mihalca et al., Reference Mihalca, Păstrav, Sándor, Deak, Gherman, Sarmaşi and Votýpka2019). Besides anecdotal references, there are no clear reports of H. longipennis on birds, so that this finding can be also considered circumstantial. Yet, the clear pattern found (preference of louse flies for pigeons) could be explained either by a higher abundance of the louse fly species typical of this particular host and/or interspecific differences in the suitability of each host species and their nests characteristics for the co-existing louse fly species (e.g. Veiga et al., Reference Veiga, De Oña, Salazar and Valera2019). Understanding the pattern of occurrence of these parasites and what factors contribute to the interspecific differences found here requires detailed knowledge of the natural history and host choice criteria of these species and goes beyond the aims of this study. Nonetheless, it is important to remark that even in situations favouring intra- and interspecific parasite transmission, clear preference patterns are evident.
Ectoparasite abundance in mixed-species colonies is mostly driven by colony composition rather than colony size or density
It has been widely acknowledged that social breeding incurs in higher risk of parasite and pathogen transmission, with larger, denser colonies, having higher prevalence and intensity of ectoparasites, even in mobile, non-contact-transmitted species (Brown and Brown, Reference Brown and Brown1986; Côté and Poulin, Reference Côté and Poulin1995; Hoi et al., Reference Hoi, Darolová, König and Krištofík1998; Kleindorfer and Dudaniec, Reference Kleindorfer and Dudaniec2009; Veiga et al., Reference Veiga, Václav and Valera2020). Most of these studies have focused on mono-specific colonies or on single parasite species, overlooking the complex multi-species associations that often occur in nature (Valera et al., Reference Valera, Casas-Crivillé and Hoi2003). The results of this study on mixed-species colonies suggest that colony size, density or richness, common characteristics used to measure host sociality, do not affect the infracommunity of ectoparasites in each host, or the abundance of the generalist Carnus. Although the correlation among these variables prevented the identification of the isolated effect of each trait, none were selected using a model-averaging approach, suggesting a lack or weak effect of these variables in our study system. Instead, host species was the main predictor for Carnus abundance. Besides colony composition, which will be discussed below, Carnus abundance was found to increase with brood size. More nestlings in a nest translates to more food resources for ectoparasites and higher heat and CO2 emissions that may facilitate nest detection. Thus, an increase in Carnus abundance with larger broods was expected (see also Veiga et al., Reference Veiga, Václav and Valera2020). In contrast to previous results (Calero-Torralbo et al., Reference Calero-Torralbo, Václav and Valera2013; Veiga et al., Reference Veiga, Václav and Valera2020), the abundance of Carnus was not influenced by sampling date – a proxy of Carnus emergence patterns. But, contrary to these studies, the results reported here consider simultaneously different host species, so the lack of a global effect of date may be related to merging host species with different breeding phenologies, number of clutches and nestling development rates. There was also no influence of type of nest except for a marginally significant decrease in clay pots. Clay pots are shallower than cavities or nest-boxes, and some of them have low (sometimes almost inexistent) amounts of detritus, which may inhibit egg deposition or larvae development by Carnus.
The main finding of this study is that in mixed-species assemblages, rather than colony size or density, it is the composition of the colony – the number of nests of the various host species – that influences the abundance of the most common ectoparasite. Carnus abundance decreased with increasing number of starling nests and increased with increasing numbers of lesser kestrels. Because Carnus mean intensity varied considerably between hosts, with lesser kestrels hosting more than three times more Carnus than starlings, starling-dominated colonies will have lower Carnus loads than lesser kestrel dominated ones. If the proportion of less competent hosts such as starlings increases in a mixed colony, then the total amount of ectoparasites will decrease. A dilution effect is occurring by ‘adding’ less competent host species, starlings (or pigeons), to the colony (Johnson and Thieltges, Reference Johnson and Thieltges2010; Civitello et al., Reference Civitello, Choen, Fatima, Halstead, Liriano, McMahon, Ortega, Sauer, Sehgal, Young and Rohr2015). Because most Carnus' life stages occur in the nest (Valera et al., Reference Valera, Casas-Crivillé and Calero-Torralbo2006a), Carnus will produce less offspring in a colony dominated by less suitable hosts than in a colony with more suitable hosts. In parallel, the number of Carnus will increase in colonies with increasing number of preferred hosts such as lesser kestrels and rollers. This suggests that increasing colony diversity would only result in a dilution effect – decreasing parasitism – if the added host species are less suitable for a given parasite. For instance, pigeons are avoided by Carnus but are preferred by P. canariensis (Veiga et al., Reference Veiga, De Oña, Salazar and Valera2019), so having pigeons in colonies may decrease Carnus abundance but increase P. canariensis in lesser kestrel nests.
The findings of this study must be considered in light of some limitations. Colony size, density, and host richness were correlated so that larger colonies also tended to have higher nest density and higher richness of hosts. This prevented the investigation of the effect of each colony trait, only allowing the comparison between smaller colonies (less dense and less rich) from larger ones (denser and richer). Increasing the sample size (which was not possible for this study due to logistics constrains) could potentially allow for the investigation of the correlation/interaction between colony traits and reveal other effects the authors were unable to detect. However, because large colonies had different host species composition – some dominated by lesser kestrels and others by starlings – this study was able to untwine the effects of colony composition on ectoparasite infestation patterns. Also, as opposed to similar studies (Veiga et al., Reference Veiga, De Oña, Salazar and Valera2019), nests in this study were not sanitized before sampling. As such, ectoparasites that spend most of their life cycle in the nest, such as carnid flies, were not removed, and so the prevalence and abundance (and their variability) found in this study may have been at least partially influenced by the outcome of previous breeding seasons (Valera et al., Reference Valera, Casas-Crivillé and Calero-Torralbo2006a). Nonetheless, results from this study suggest that the host species occupying the focal nest in the previous year had no effect (variable not selected) on the ectoparasite infracommunity or on Carnus abundance. The influence of the abundance of ectoparasites during the previous season may be diluted for several reasons. For instance, C. hemapterus – arguably the most nest-based ectoparasite from the ones addressed – was found to rapidly colonize new (and clean) nests and to discriminate between potential hosts, favouring some of them (Veiga et al., Reference Veiga, De Oña, Salazar and Valera2019, Reference Veiga, Václav and Valera2020). Moreover, insect predation after the breeding season may decrease the abundance of carnid pupae (Salido et al., Reference Salido, Veiga, Reyes-Lopez, Nieves-Aldrey and Valera2021). These facts suggest that our approach may have had little impact on the results found for mobile ectoparasites (carnid flies and louse flies) and for parasites spending all their life cycle on the host's body (e.g. lice). On the other hand, by examining uncleaned nests, this study reflects more closely the natural conditions than studies using sanitized nests, and thus our results are likely to apply to birds breeding in natural assemblages (Møller, Reference Møller1989). Finally, this study examines ectoparasite groups instead of individual species. This limits our capacity to establish species-specific parasite–host relationships, but it does not preclude disentangling the effects of colony traits on the general patterns of ectoparasite infestations.
Final considerations
Previous studies on monospecific colonies have found a positive correlation between colony size and density and C. hemapterus infestations (Hoi et al., Reference Hoi, Darolová, König and Krištofík1998, Reference Hoi, Krištofík, Darolová and Hoi2010; Veiga et al., Reference Veiga, Václav and Valera2020, but see Liker et al., Reference Liker, Márkus, Vozár, Zemankovics and Rózsa2001). The current study in mixed-species colonies revealed that colony traits such as size, density, richness, or composition, had no effect on ectoparasite presence, with each avian host maintaining a distinct ectoparasite infracommunity. It also revealed that colony composition, rather than colony size or density, influenced the infestation of the most common ectoparasite in these assemblages. Carnus can be found from North America to Europe and Asia infesting a wide range of bird species, so the implications of this study go beyond our study area (Grimaldi, Reference Grimaldi1997; Brake, Reference Brake2011; Ganbold et al., Reference Ganbold, Azua, Munkhbayar, Khuderchuluun, Paek, Purevee, Chuluunbat and Reading2020). The association between farmland buildings or artificial nests and secondary cavity nesters such as lesser kestrels and rollers is common in other European counties, such as Spain and Italy (Campobello et al., Reference Campobello, Sarà and Hare2012; Negro et al., Reference Negro, Prenda, Ferrero, Rodríguez and Reig-Ferrer2020). Similar natural species assemblages occur in sandstone cliffs and bridges (e.g. in south Spain), where many species, including the ones studied here (and others such as common kestrels, little owls, jackdaws, bee-eaters Merops apiaster and rock sparrows Petronia petronia) coexist and re-use the same natural and man-made cavities (Valera et al., Reference Valera, Casas-Crivillé and Hoi2003; Veiga et al., Reference Veiga, Václav and Valera2020). Lesser kestrel colonies throughout Europe that rely on man-made buildings are also likely comprised of other species (Blanco and Tella, Reference Blanco and Tella1997; Campobello et al., Reference Campobello, Sarà and Hare2012). Densities in these ‘natural scenarios’ are likely lower than those in artificial breeding towers, but it may depend on ecological circumstances such as availability of nest-sites. Our results that larger colonies do not necessarily translate into higher risk of ectoparasite transmission, and that this effect is influenced by the species composition of the colonies, are likely applicable to other mixed-species colonies of birds or even other non-bird mixed-species groups (Goodale et al., Reference Goodale, Sridhar, Sieving, Bangal, Colorado Z, Farine, Heymann, Jones, Krams, Martínez, Montaño-Centelles, Muñoz, Srinivasan, Theo and Shanker2020). Yet, the exact outcome may vary depending on the specific parasites and hosts forming the assemblages. The interpretation of the ecological relationships between parasites and hosts should consider the interactions occurring among different hosts and their parasites and should be studied as they occur in nature, i.e. considering mixed-species assemblages, which are often overlooked despite being widespread. More studies on the natural history of ectoparasites and on host–parasite interactions in multi-species group living are clearly needed to fully understand the complex relationships between parasites and sociality.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182021000470.
Acknowledgements
We would like to thank Liga para a Proteção da Natureza (LPN) for providing access to the colonies within the Castro Verde Special Protection Area, and to Instituto da Conservação da Natureza e Florestas (ICNF) for giving us the permission to sample bird's nests. We thank all contributors who helped during fieldwork and data collection. We would also like to thank the editor Helene Carabin and three anonymous reviewers for their valuable suggestions, which have greatly improved this manuscript.
Author contribution
All authors were responsible for conceiving and designing the study. J. G. and I. C. conducted data collection. J. G. and J. V. performed statistical analyses. J. G. wrote the manuscript. All authors reviewed and contributed to the final paper.
Financial support
This study was supported by cE3c (UIBD/00329/2020) and InBIO (UID/BIA/50027/2013), to FCT through national funds. I. C. was supported by a contract DL57/2016/CP1440/CT0023 and J. G. by a doctoral grant (PD/BD/128366/2017) from the Portuguese Foundation for Science and Technology (FCT). F. V. received financial support from the project PGC2018-097426-B-C22 (MCIU/AEI/FEDER, UE). J. V. was funded by a predoctoral grant (BES-2015-075951) of the Spanish Ministry of Economy, Industry and Competitiveness. The funding sources had no direct involvement in the study design or in the collection, analysis and interpretation of data.
Conflict of interest
The authors declare there are no conflicts of interest.
Ethical standards
Trapping and handling of birds during this study was approved by Instituto da Conservação da Natureza e Florestas (License 547/2018/CAPT).