INTRODUCTION
Toxoplasma gondii, Neospora spp., Sarcocystis spp., Hammondia spp. and Besnoitia besnoiti are closely related tissue cyst-forming parasites that belong to the family Sarcocystidae (Carreno et al. Reference Carreno, Schnitzler, Jeffries, Tenter, Johnson and Barta1998; Mugridge et al. Reference Mugridge, Morrison, Jakel, Heckeroth, Tenter and Johnson2000). The complete life cycles of Sarcocystidae organisms are complex and involve several parasite stages in definitive and intermediate hosts. In species with known life cycles, carnivore or omnivore definitive hosts harbour sexual reproduction of the parasites in intestinal epithelium. They may shed large numbers of parasite oocysts or sporocysts in their feces. Intermediate hosts acquire infection upon ingestion of sporulated oocysts or sporocysts in food or water. Sporozoites invade intestinal epithelial cells of the intermediate hosts and spread to other tissues as tachyzoites or merozoites. These latter stages may encyst as slow multiplying forms, called bradyzoites. Ingestion of tissue cysts by carnivorism is the main route of infection for definitive hosts, which culminates with the formation of oocysts in their intestinal epithelium (Levine and Ivens, Reference Levine and Ivens1981).
Among the cyst-forming parasites, T. gondii is the most studied. It induces disease in a wide range of warm-blooded animals, including humans (Tenter et al. Reference Tenter, Heckeroth and Weiss2000), and causes abortion in livestock, especially in sheep and goats (Buxton, Reference Buxton1998). Neospora caninum was originally described in dogs (Bjerkas et al. Reference Bjerkas, Mohn and Presthus1984; Dubey et al. Reference Dubey, Carpenter, Speer, Topper and Uggla1988a ), but it gradually gained more attention from the scientific community as a major cause of neonatal mortality and abortion in mainly cattle but also other ruminants (O'Toole and Jeffrey, Reference O'Toole and Jeffrey1987; Parish et al. Reference Parish, Maag-Miller, Besser, Weidner, McElwain, Knowles and Leathers1987; Anderson et al. Reference Anderson, Blanchard, Barr, Dubey, Hoffman and Conrad1991), besides causing neuromuscular disease in dogs (Ruehlmann et al. Reference Ruehlmann, Podell, Oglesbee and Dubey1995). Neospora hughesi was proposed as a new species in the genus Neospora and has been associated with myeloencephalitis in horses (Marsh et al. Reference Marsh, Barr, Packham and Conrad1998). The genus Sarcocystis possesses more than 100 species, with cattle as intermediate hosts of at least three species (S. cruzi, S. hirsuta and S. hominis). Among these, only S. cruzi is mildly pathogenic for cattle and generally non-pathogenic for its definitive host (dog) (reviewed by Dubey and Lindsay, Reference Dubey and Lindsay2006). Additional Sarcocystis spp. have been observed in bovine tissues, but their nomenclatures are still in debate (Dubey et al. Reference Dubey, More, van Wilpe, Calero-Bernal, Verma and Schares2016; Gjerde, Reference Gjerde2016). Three parasite species compose the genus Hammondia (H. hammondi, H. heydorni and H. triffittae), which have no known association with disease in humans or in naturally infected animals. However, Hammondia spp. are closely related to T. gondii and N. caninum (Mugridge et al. Reference Mugridge, Morrison, Jakel, Heckeroth, Tenter and Johnson2000), so diagnostic methods need to discriminate between infections caused by these parasites. Besnoitia besnoiti causes a debilitating disease mainly characterized by both a cutaneous and systemic manifestation (Alvarez-Garcia et al. Reference Alvarez-Garcia, Frey, Mora and Schares2013). Reproductive abnormalities in cattle may also occur, such as infertility in bulls and abortion when cows are infected during pregnancy (Cortes et al. Reference Cortes, Leitao, Gottstein and Hemphill2014). Bovine besnoitiosis was reported first more than a century ago in Southern France and Portugal, but the parasite has spread to several European countries during the last 10 years and besnoitiosis is now considered as a re-emerging disease in cattle, at least in Europe (Alvarez-Garcia et al. Reference Alvarez-Garcia, Frey, Mora and Schares2013).
Toxoplasma gondii and H. hammondi have cats as definitive hosts, which shed morphologically indistinguishable oocysts in their feces. Dogs and certain canid species serve as definitive hosts for N. caninum (McAllister et al. Reference McAllister, Dubey, Lindsay, Jolley, Wills and McGuire1998; Gondim et al. Reference Gondim, McAllister, Pitt and Zemlicka2004; King et al. Reference King, Slapeta, Jenkins, Al-Qassab, Ellis and Windsor2010; Dubey et al. Reference Dubey, Jenkins, Rajendran, Miska, Ferreira, Martins, Kwok and Choudhary2011) and H. heydorni (Blagburn et al. Reference Blagburn, Lindsay, Swango, Pidgeon and Braund1988; Slapeta et al. Reference Slapeta, Koudela, Votypka, Modry, Horejs and Lukes2002; Soares et al. Reference Soares, Cortez, Gennari, Sercundes, Keid and Pena2009). Hammondia triffittae has two species of wild canids (red fox and arctic fox) as definitive hosts (Gjerde and Dahlgren, Reference Gjerde and Dahlgren2011). Three Sarcocystis spp. from cattle, S. cruzi, S. hirsuta and S. hominis, have dogs, cats, and primates as definitive hosts, respectively (reviewed by Gjerde, Reference Gjerde2016). Besnoitia besnoiti is suspected to have a carnivore as definitive host, but so far no animal has been identified shedding oocysts of the parasite by natural or experimental infections (Basso et al. Reference Basso, Schares, Gollnick, Rutten and Deplazes2011).
Infections caused by T. gondii, Hammondia spp., Neospora spp., Sarcocystis spp. and B. besnoiti are assessed by a great variety of diagnostic tools, depending on the purpose of the analysis and available biological sample. In clinically affected individuals, detection of parasite-specific antibodies in serum or other body fluids is the most commonly employed diagnostic approach, except for Hammondia spp., and Sarcocystis spp. from cattle. Continuous cultivation of bovine Sarcocystis spp., like S. cruzi, is difficult (Andrews et al. Reference Andrews, Fayer and Dubey1990). To date, Hammondia spp. cannot be continuously grown in cell culture, which impedes production of parasite antigens needed to produce serologic tests for these parasites (Riahi et al. Reference Riahi, Darde, Bouteille, Leboutet and Pestre-Alexandre1995; Schares et al. Reference Schares, Meyer, Barwald, Conraths, Riebe, Bohne, Rohn and Peters2003; Gondim et al. Reference Gondim, Meyer, Peters, Rezende-Gondim, Vrhovec, Pantchev, Bauer, Conraths and Schares2015). In this review, serologic cross-reactivity is reviewed in detail among infections caused by T. gondii, Hammondia spp., Neospora spp., Sarcocystis spp. and B. besnoiti. Special emphasis is put on serologic cross-reactivity among animals infected with N. caninum and related pathogens. Further consideration is given to the discovery and production of species-specific and stage-specific antigens, which promise to improve diagnostic specificity and may enable discrimination between different modes of parasite acquisition.
SEROLOGY FOR T. GONDII AND CROSS-REACTIVITY WITH RELATED PATHOGENS
Toxoplasma gondii vs H. hammondi
During the first decades after T. gondii was discovered, several scientists have attempted to develop serologic tests with high sensitivity and specificity to diagnose T. gondii infection, as well as to understand the antigenic composition of the parasite. The development and improvement of serological tests, such as the Sabin–Feldman dye test (DT) (Sabin and Feldman, Reference Sabin and Feldman1948; Beverley and Beattie, Reference Beverley and Beattie1952), direct agglutination test (Fulton and Turk, Reference Fulton and Turk1959; Desmonts and Remington, Reference Desmonts and Remington1980), complement fixation test (CFT) (Sabin, Reference Sabin1949), enzyme-linked immunosorbent assay (ELISA) (Walls et al. Reference Walls, Bullock and English1977), immunofluorescence antibody test (IFAT) (Kelen et al. Reference Kelen, Ayllon-Leindl and Labzoffsky1962), indirect haemagglutination test (IHA) (Lunde and Jacobs, Reference Lunde and Jacobs1958) and Western blot (WB) (Araujo et al. Reference Araujo, Dubey and Remington1984) favoured a great advance in the study of toxoplasmosis.
Cross-immunity studies between T. gondii and H. hammondi
Mice and hamsters that were experimentally infected with H. hammondi (CR-4 strain) oocysts developed immunity and did not die after challenged with lethal doses of oocysts from a mouse-virulent T. gondii strain (M-7741 strain) (Frenkel and Dubey, Reference Frenkel and Dubey1975). In contrast, cats that were experimentally infected with H. hammondi and shed oocysts were not immunized against excretion of T. gondii oocysts (Frenkel and Dubey, Reference Frenkel and Dubey1975). An additional study approached cross-immunity between T. gondii and H. hammondi, by using six H. hammondi strains in an infection model of mice and hamsters (Christie and Dubey, Reference Christie and Dubey1977). The authors observed that 103 of 108 mice that were orally inoculated with H. hammondi oocysts survived a lethal challenge dose (105 oocysts) of T. gondii (strain M-7741). The H. hammondi-inoculated hamsters also developed immunity against a lethal dose of T. gondii oocysts, but this immunity was variable depending on the H. hammondi strain. The two most immunogenic H. hammondi strains conferred protection to fatal toxoplasmosis in 100 and 83% of the hamsters, respectively, after challenging with T. gondii oocysts (Christie and Dubey, Reference Christie and Dubey1977).
Immunization of goats with H. hammondi had a protective effect against abortion induced by T. gondii; however, the immunization did not prevent transplacental transmission of T. gondii in pregnant does (Munday and Dubey, Reference Munday and Dubey1988). Partial immunity against toxoplasmosis was also obtained in Tamar wallabies (Macropus eugenii), that were orally infected with 1 × 105 oocysts of H. hammondi (Reddacliff et al. Reference Reddacliff, Parker, Dubey, Nicholls, Johnson and Cooper1993).
Serologic cross-reactivity between T. gondii and H. hammondi
When H. hammondi was first described, some rodent species experimentally infected with this parasite developed cross-reacting antibodies against T. gondii antigens in the DT (Frenkel and Dubey, Reference Frenkel and Dubey1975). Further studies were carried out and confirmed that serologic cross-reactivity between T. gondii and H. hammondi occurred with sera from other animals, besides rodents. Weiland et al. (Reference Weiland, Rommel and von Seyerl1979) investigated cross-reactivity between T. gondii and H. hammondi in four animal species (120 mice, six dogs, six rabbits and six pigs) by using five serological tests (DT, CFT, ELISA, IFAT and IHA). Half of the animals were orally inoculated with T. gondii oocysts and the other half received H. hammondi oocysts by the same route. Sera from H. hammondi-infected mice reacted with T. gondii antigens in three tests (DT, ELISA and CFT). Sera from dogs infected with H. hammondi recognized T. gondii antigens by DT and ELISA. Sera from rabbits exhibited cross-reaction between the two parasites by ELISA. The sera of pigs infected with H. hammondi did not cross-react with T. gondii in any of the five serological tests. In this study, the IFAT was considered the most Toxoplasma-specific method. During the course of infection, the animals infected with T. gondii presented higher titres in the tests when compared with those infected with H. hammondi (Weiland et al. Reference Weiland, Rommel and von Seyerl1979). Munday and Dubey (Reference Munday and Dubey1986) observed that sheep that were inoculated with H. hammondi oocysts presented cross-reactivity with T. gondii antigen by IFAT. Before infection with H. hammondi, the sheep had no detectable antibodies to T. gondii. After oral inoculation with H. hammondi oocysts, the animals presented antibody titres of 1:16 to T. gondii by IFAT. Goats infected with H. hammondi were shown to produce antibodies against T. gondii tested by DT, with titres up to 1:64 (Dubey, Reference Dubey1981).
The antigenic similarity between T. gondii and H. hammondi was investigated using sera from experimentally infected mice (Araujo et al. Reference Araujo, Dubey and Remington1984). The authors employed T. gondii tachyzoites (RH strain) for two antigen detection procedures: (1) the antigen was labelled with 125I, immune-precipitated with sera from T. gondii or H. hammondi-infected mice, run by SDS–polyacrylamide gel electrophoresis (SDS–PAGE) and autoradiographed; (2) the antigen was lysed, separated by SDS–PAGE and tested by immunoblot. The sera from mice that were orally inoculated with H. hammondi oocysts recognized T. gondii antigens of MW 92·5 kDa (two antigens), 66·2 kDa, between 66·2 and 45 kDa, and between 31 and 21·5 kDa; compared with the T. gondii-positive reaction only a T. gondii antigen of 21·5 kDa was not recognized by the H. hammondi-positive mouse serum (Araujo et al. Reference Araujo, Dubey and Remington1984). This study confirmed that T. gondii and H. hammondi have similar antigenic components in their tachyzoites.
To facilitate scientific communication among research groups and laboratories, Sibley et al. (Reference Sibley, Pfefferkorn and Boothroyd1991) proposed a nomenclature system for naming mutants, genes and gene products of T. gondii, which was based on the system used for the yeast Saccharomyces cerevisiae. As an example, the surface protein (P30) was designated SAG1, which is the product of the SAG1 gene. However, as it was observed more recently that SAG1 genes belong to a superfamily of related genes, named SRS (SAG1-related sequences), which encode a superfamily of structurally related surface proteins from T. gondii, the name of SAG1 (P30) has been changed to SRS29B (Wasmuth et al. Reference Wasmuth, Pszenny, Haile, Jansen, Gast, Sher, Boyle, Boulanger, Parkinson and Grigg2012).
Riahi et al. (Reference Riahi, Bouteille and Darde1998) studied the antigenic similarity of T. gondii and H. hammondi employing five monoclonal antibodies (MAbs) against T. gondii surface antigens and a polyclonal mouse serum to H. hammondi. In order to produce enough H. hammondi antigen, the authors used an in vitro model that allows the production of H. hammondi cysts up to 3 months in cell culture (Riahi et al. Reference Riahi, Darde, Bouteille, Leboutet and Pestre-Alexandre1995). The cyst formation, confirmed by ultrastructural characteristics of the organism, started from 6 days after sporozoites were inoculated into feline kidney cells (CRFK). At 4 days of infection, the authors assumed that the multiplying H. hammondi zoites were tachyzoites (Riahi et al. Reference Riahi, Bouteille and Darde1998).
Tachyzoite antigens from T. gondii and H. hammondi were tested by IFAT and WB. By combining the two serologic techniques (IFAT and WB), five T. gondii antigens (MW of 30, 32, 35, 66 and 90 kDa) were recognized using polyclonal anti-H. hammondi serum. An interesting finding obtained by Riahi et al. (Reference Riahi, Bouteille and Darde1998) was the recognition of the SAG1 (SRS29B) antigen by the anti-H. hammondi serum, as this protein is a major antigen of T. gondii (Burg et al. Reference Burg, Perelman, Kasper, Ware and Boothroyd1988) and had been considered to be a specific marker for the parasite (Mineo et al. Reference Mineo, McLeod, Mack, Smith, Khan, Ely and Kasper1993). Despite the antigenic similarity between T. gondii and H. hammondi, MAbs targeted against H. hammondi antigens were produced, and five of them did not cross-react with T. gondii antigens (Riahi et al. Reference Riahi, Leboutet, Labrousse, Bouteille and Darde2000). These findings are promising for the characterization of H. hammondi-specific antigens or epitopes, which could enable development of serologic tests for this parasite that would not cross-react with T. gondii.
The genome of a German strain of H. hammondi was sequenced and the genomic synteny between this parasite and T. gondii was higher than 95% (Walzer et al. Reference Walzer, Adomako-Ankomah, Dam, Herrmann, Schares, Dubey and Boyle2013). It was found that orthologues of key T. gondii mouse virulence genes are functionally conserved in H. hammondi, but these data were not enough to explain the phenotypic differences observed between both parasites (Walzer et al. Reference Walzer, Adomako-Ankomah, Dam, Herrmann, Schares, Dubey and Boyle2013). In a recent work, the genomes of 62 strains of T. gondii were compared with those from H. hammondi, N. caninum and Sarcocystis neurona (Lorenzi et al. Reference Lorenzi, Khan, Behnke, Namasivayam, Swapna, Hadjithomas, Karamycheva, Pinney, Brunk, Ajioka, Ajzenberg, Boothroyd, Boyle, Darde, Diaz-Miranda, Dubey, Fritz, Gennari, Gregory, Kim, Saeij, Su, White, Zhu, Howe, Rosenthal, Grigg, Parkinson, Liu, Kissinger, Roos and David Sibley2016); these authors demonstrated that T. gondii possesses an expansion of parasite-specific secretory pathogenesis determinants (SPDs) when compared with the three latter parasites. The SPDs encompass genes encoding secretory proteins from micronemes, dense granules, rhoptries and surface antigens, whose expansion and diversity are associated with the patterns of transmission, host range and pathogenicity of T. gondii (Lorenzi et al. Reference Lorenzi, Khan, Behnke, Namasivayam, Swapna, Hadjithomas, Karamycheva, Pinney, Brunk, Ajioka, Ajzenberg, Boothroyd, Boyle, Darde, Diaz-Miranda, Dubey, Fritz, Gennari, Gregory, Kim, Saeij, Su, White, Zhu, Howe, Rosenthal, Grigg, Parkinson, Liu, Kissinger, Roos and David Sibley2016). It was reported that T. gondii shares a high number of orthologues with H. hammondi (7095) and N. caninum (6308) (Lorenzi et al. Reference Lorenzi, Khan, Behnke, Namasivayam, Swapna, Hadjithomas, Karamycheva, Pinney, Brunk, Ajioka, Ajzenberg, Boothroyd, Boyle, Darde, Diaz-Miranda, Dubey, Fritz, Gennari, Gregory, Kim, Saeij, Su, White, Zhu, Howe, Rosenthal, Grigg, Parkinson, Liu, Kissinger, Roos and David Sibley2016).
The search for more practical and efficient methods to detect specific antibodies against T. gondii pushed the establishment of tests based on antigenic fractions, which are gradually replacing traditional tests based on whole organisms (agglutination, DT and IFAT) or total extracts of parasite antigens. Recognized immunodominant antigens of T. gondii, including the tachyzoite surface antigens SAG1 (p30) (Kasper, Reference Kasper1987; Mineo et al. Reference Mineo, McLeod, Mack, Smith, Khan, Ely and Kasper1993), SAG2 (p22) (Prince et al. Reference Prince, Auer, Huskinson, Parmley, Araujo and Remington1990), SAG3 (P43) (Cesbron-Delauw et al. Reference Cesbron-Delauw, Tomavo, Beauchamps, Fourmaux, Camus, Capron and Dubremetz1994), SAG4 (p18) (Odberg-Ferragut et al. Reference Odberg-Ferragut, Soete, Engels, Samyn, Loyens, Van Beeumen, Camus and Dubremetz1996) and several other proteins from dense granules, rhoptries and micronemes are being produced as recombinant proteins (reviewed by Holec-Gasior, Reference Holec-Gasior2013).
So far, it is not known whether humans may be infected with H. hammondi, and in case it happens, the possibility of serologic cross-reactivity with T. gondii antigens cannot be ruled out. Cats seem to shed T. gondii and H. hammondi in similar proportions as reported in a recent study from Germany (Schares et al. Reference Schares, Ziller, Herrmann, Globokar, Pantchev and Conraths2016), and humans and animals of many species are potentially exposed to H. hammondi. The establishment of H. hammondi infections in most animal species has not been rigorously investigated; however, the life cycle of H. hammondi seems to lack avian hosts (Dubey and Sreekumar, Reference Dubey and Sreekumar2003).
Toxoplasma gondii vs N. caninum
The serologic differentiation between T. gondii and H. hammondi infections has clinical and epidemiological relevance. Since H. hammondi is not known to induce disease in animals or humans, the major concern about the serologic differentiation of these parasites seems to be to avoid T. gondii false-positive results in individuals potentially infected with H. hammondi. The identification of N. caninum (Bjerkas et al. Reference Bjerkas, Mohn and Presthus1984; Dubey et al. Reference Dubey, Carpenter, Speer, Topper and Uggla1988a ) imposed a new challenge for the scientific community, because this non-zoonotic Toxoplasmatinae parasite is able to infect and cause disease in some mammalian animals that are also susceptible to T. gondii infection (Buxton, Reference Buxton1998). The comparison of the whole-genome sequences from T. gondii, H. hammondi and N. caninum showed that these three related tissue-cyst-forming coccidian parasites have a similar total genome size of 62–65 Mb, and many similar orthologous groups of proteins involved in key biological functions (Lorenzi et al. Reference Lorenzi, Khan, Behnke, Namasivayam, Swapna, Hadjithomas, Karamycheva, Pinney, Brunk, Ajioka, Ajzenberg, Boothroyd, Boyle, Darde, Diaz-Miranda, Dubey, Fritz, Gennari, Gregory, Kim, Saeij, Su, White, Zhu, Howe, Rosenthal, Grigg, Parkinson, Liu, Kissinger, Roos and David Sibley2016).
Neospora caninum can be maintained as tachyzoites in cell culture (Dubey et al. Reference Dubey, Hattel, Lindsay and Topper1988b ), which has enabled the development of a range of serological tests and antibodies against the parasite (Björkman and Uggla, Reference Björkman and Uggla1999). Neosporosis research was accelerated by the prior accumulation of knowledge about and procedures for T. gondii.
The nomenclature system adopted for mutants, genes and gene products of T. gondii (Sibley et al. Reference Sibley, Pfefferkorn and Boothroyd1991) was proposed for N. caninum (Howe and Sibley, Reference Howe and Sibley1999). In the comparison of T. gondii and N. caninum homologous antigens (e.g. SAG1), the use of a Tg or Nc prefix was recommended to distinguish these gene products (e.g. TgSAG1 and NcSAG1) (Howe and Sibley, Reference Howe and Sibley1999). Also, a cluster of cell-surface genes was found in N. caninum, as described for T. gondii, and these gene products were re-named as members of the SRS superfamily (Wasmuth et al. Reference Wasmuth, Pszenny, Haile, Jansen, Gast, Sher, Boyle, Boulanger, Parkinson and Grigg2012).
Cross-immunity studies between T. gondii and N. caninum
Cross-protection of mice immunized with N. caninum and challenged with T. gondii is probably T. gondii strain- and dose-dependent. In one study, mice were immunized with N. caninum and died after challenging with the highly virulent RH strain of T. gondii (Lindsay et al. Reference Lindsay, Blagburn and Dubey1990). When mice were immunized with N. caninum and challenged with a less-virulent T. gondii strain (PLK, a clone from the ME49 strain), the animals had 100% protection against death; immunization with a higher dose of N. caninum tachyzoites improved protection against T. gondii-induced disease (Kasper and Khan, Reference Kasper and Khan1998). Similar levels of protection were observed when mice were immunized with N. caninum tachyzoites and then challenged with oocysts of T. gondii from a moderately virulent strain (Lindsay et al. Reference Lindsay, Lenz, Dykstra, Blagburn and Dubey1998). In another study, pregnant sheep were immunized with a T. gondii sheep vaccine (Toxovax®, Intervet, Cambridge, UK) and challenged with a high dose of N. caninum tachyzoites (107 tachyzoites per animal) at 90 days of gestation (Innes et al. Reference Innes, Lundén, Esteban, Marks, Maley, Wright, Rae, Harkins, Vermeulen, McKendrick and Buxton2001). No protection against fetal death was observed. The authors speculated that if the sheep were challenged with a lower dose of N. caninum tachyzoites, there perhaps would be some degree of cross-immunity (Innes et al. Reference Innes, Lundén, Esteban, Marks, Maley, Wright, Rae, Harkins, Vermeulen, McKendrick and Buxton2001). The confirmation of cross-immunity between T. gondii and N. caninum in some studies suggests that these parasites share antigens which may also be involved in serologic cross-reactivity.
Serologic cross-reactivity between T. gondii and N. caninum
In the initial observation of N. caninum in dogs, sera from five animals that were naturally infected with the parasite tested negative for T. gondii by the DT (Bjerkas et al. Reference Bjerkas, Mohn and Presthus1984). In addition, dogs that were naturally or experimentally infected with N. caninum did not cross-react to T. gondii by IFAT, when using 1:50 dilutions as cutoff (Dubey et al. Reference Dubey, Hattel, Lindsay and Topper1988b ). Accordingly, the same or higher dilution cutoffs by IFAT have been found to be appropriate to avoid cross-reactivity between N. caninum and T. gondii in serum samples from different hosts (Lobato et al. Reference Lobato, Silva, Mineo, Amaral, Segundo, Costa-Cruz, Ferreira, Borges and Mineo2006; Silva et al. Reference Silva, Lobato, Mineo and Mineo2007; Benetti et al. Reference Benetti, Schein, dos Santos, Toniollo, da Costa, Mineo, Lobato, de Oliveira Silva and Gennari2009). Apical reactions, i.e. reactions limited to the apex of the parasite were regarded as non-specific in N. caninum IFAT. Cross-reactivity with apical antigens is potentially caused by the high conservation of antigens in the apical organelles of a variety of Apicomplexan parasites, including T. gondii. In contrast, a complete peripheral fluorescence of the parasite was considered as a positive response (Pare et al. Reference Pare, Hietala and Thurmond1995b ).
Cross-reactive antigens between N. caninum and T. gondii have been observed by immunohistochemistry using tissues of naturally- or experimentally infected animals. A rabbit anti-N. caninum serum cross-reacted with T. gondii in tissue sections from mice (Barr et al. Reference Barr, Conrad, Dubey and Anderson1991). A bradyzoite antigen from T. gondii, designated as BAG1 (synonymous to BAG5) (Weiss et al. Reference Weiss, LaPlace, Tanowitz and Wittner1992; Parmley et al. Reference Parmley, Weiss and Yang1995), was used to produce hyperimmune serum in rabbit, which cross-reacted with bradyzoites of N. caninum (McAllister et al. Reference McAllister, Parmley, Weiss, Welch and McGuire1996). Polyclonal sera against T. gondii tachyzoites induced strong cross-reactivity with N. caninum tachyzoites by immunohistochemistry (Sundermann et al. Reference Sundermann, Estridge, Branton, Bridgman and Lindsay1997). The use of MAbs specific to T. gondii (Sundermann et al. Reference Sundermann, Estridge, Branton, Bridgman and Lindsay1997) or a combination of two MAbs specific to N. caninum (Uzêda et al. Reference Uzêda, Schares, Ortega-Mora, Madruga, Aguado-Martinez, Corbellini, Driemeier and Gondim2013), were demonstrated to avoid immunohistological cross-reactivity between these protozoa.
Although species-specific MAbs have been developed, serologic cross-reactions between T. gondii and N. caninum have been shown to occur also by means of MAbs. Sundermann et al. (Reference Sundermann, Estridge, Branton, Bridgman and Lindsay1997) generated MAbs against T. gondii tachyzoites and observed among 26 MAbs tested by IFAT, five antibodies that cross-reacted with N. caninum tachyzoites. Kobayashi et al. (Reference Kobayashi, Narabu, Yanai, Hatano, Ito, Imai and Ike2013) cloned the NcBAG1 gene and generated MAbs against its recombinant protein, which recognized TgBAG1. Liao et al. (Reference Liao, Xuan, Huang, Shirafuji, Fukumoto, Hirata, Suzuki and Fujisaki2005a ) produced 384 MAbs against N. caninum by immunizing mice with N. caninum tachyzoites; 10 of the 384 MAbs were also reactive against T. gondii tachyzoites. Similarly, Sohn et al. (Reference Sohn, Cheng, Drummond, Peng, Vermont, Xia, Cheng, Wastling and Bradley2011) developed 46 MAbs using a mouse immunized with a mixed fraction of N. caninum organelles and some of the MAbs cross-reacted with T. gondii. MAbs generated to oocyst antigens of T. gondii cross-reacted by immunofluorescence with the sporocyst wall (Dumetre and Darde, Reference Dumetre and Darde2007) and tissue the cyst wall of N. caninum (Gondim et al. Reference Gondim, Wolf, Vrhovec, Pantchev, Bauer, Langenmayer, Bohne, Teifke, Dubey, Conraths and Schares2016). These findings show that cross-reactive antigens between T. gondii and N. caninum are present in tachyzoites, tissue cysts and oocysts of these parasites.
Affinity-purified antibodies raised against a 38 kDa microneme-associated protein of N. caninum (NcMIC3) also recognized a 45 kDa protein in tachyzoite extracts of T. gondii (Sonda et al. Reference Sonda, Fuchs, Gottstein and Hemphill2000). MAbs raised against T. gondii reacted with N. caninum tachyzoites by IFAT, but only at low titres (10–40) (Latif and Jakubek, Reference Latif and Jakubek2008). A number of reports demonstrated the usefulness of MAbs against T. gondii, which do not cross-react with N. caninum (Baszler et al. Reference Baszler, Adams, Vander-Schalie, Mathison and Kostovic2001; Uchida et al. Reference Uchida, Ike, Kurotaki, Ito and Imai2004; Srinivasan et al. Reference Srinivasan, Baszler, Vonlaufen, Leepin, Sanderson, Wastling and Hemphill2006; Cunha-Junior et al. Reference Cunha-Junior, Silva, Silva, Souza, Souza, Prudencio, Pirovani, Cezar, Barbosa, Goulart and Mineo2010).
Certain surface antigens of N. caninum, although not identical to those from T. gondii, were homologous to them (Hemphill et al. Reference Hemphill, Felleisen, Connolly, Gottstein, Hentrich and Muller1997; Howe et al. Reference Howe, Crawford, Lindsay and Sibley1998; Howe and Sibley, Reference Howe and Sibley1999). Two surface antigens of N. caninum, similar to SAG1 and SRS2 from T. gondii, were called NcSAG1 and NcSRS2. MAbs against NcSAG1 (6C11, Ncmab-4) did not cross react with T. gondii (Björkman and Hemphill, Reference Björkman and Hemphill1998; Howe et al. Reference Howe, Crawford, Lindsay and Sibley1998). Evaluation of several MAbs against NcSRS2 (5H5, Ncmab-10, 5·2·15) revealed no cross-reactions with T. gondii antigens in immunoblot (Björkman and Hemphill, Reference Björkman and Hemphill1998; Howe et al. Reference Howe, Crawford, Lindsay and Sibley1998; Schares et al. Reference Schares, Dubremetz, Dubey, Barwald, Loyens and Conraths1999a ).
Antigens of N. caninum tested by WB using monoclonal or polyclonal antibodies, resulted in the identification of a limited number of specific immunodominant bands (often referred to as ‘immunodominant antigens’) and other less reactive bands (Table 1). The comparison of these antigens based on their molecular weights is difficult, as differences on the SDS-PAGE conditions, especially the use of reducing or non-reducing conditions, cause variation in the estimated molecular weights. Most likely, some of these immunodominant bands observed in WBs may represent more than a single protein.
Table 1. Immunodominant bands recognized by Neospora caninum-infected or immunized animals in tachyzoite antigen

a Cross-reacting immunodominant antigen bands underlined.
In WB, non-reduced antigens exhibit much stronger reactivity than reduced antigens (Barta and Dubey, Reference Barta and Dubey1992) which is a clear indication that most of the epitopes recognized on these antigens are conformational epitopes. Under non-reduced conditions a large number of researchers observed mainly four areas with specific immunodominant bands in N. caninum tachyzoite antigen: 14–19 kDa, 29–32 kDa, 30–36 kDa, and 36–40 kDA. However, other areas with major bands of reactions were also observed with non-reduced antigens, but differed widely between studies (Table 1).
Sera from animals immunized with recombinant forms of the major N. caninum antigens NcSAG1 and NcSRS2 provided evidence that these antigens are among those recognized in the range 29–32 kDa and 36–40 kDA bands (Howe et al. Reference Howe, Crawford, Lindsay and Sibley1998) (Table 2).
Table 2. Neospora caninum-recombinant antigens and cross-reactions tested against Toxoplasma gondii and related protozoan parasites

Under reduced conditions there is a dominant band between 17–19 kDa which most likely represent reactions against NcGRA7 (Alvarez-Garcia et al. Reference Alvarez-Garcia, Pitarch, Zaballos, Fernandez-Garcia, Gil, Gomez-Bautista, Aguado-Martinez and Ortega-Mora2007) and other antigens (Table 2). However, as demonstrated by MAbs (4·7·12; Ncmab-7) in combination with immunoprecipitation, surface biotinylation and immuno-electron microscopy, a surface antigen might also be among those migrating in WB at 17–19 kDa under reduced conditions (Björkman and Hemphill, Reference Björkman and Hemphill1998; Schares et al. Reference Schares, Dubremetz, Dubey, Barwald, Loyens and Conraths1999a ). Further immunodominant banding areas are at 34–36 and 37–46 kDa, eventually also representing reactions to NcSAG1, NcGRA6, NcGRA7 and NcSRS2 (Table 2). When tested with polyclonal antibodies against T. gondii, only minor reactions were observed with antigen bands regarded as specific for N. caninum in WB (Barta and Dubey, Reference Barta and Dubey1992; Björkman et al. Reference Björkman, Lunden, Holmdahl, Barber, Trees and Uggla1994, Reference Björkman, Holmdahl and Uggla1997).
For the diagnosis of T. gondii infection in veterinary investigations, the use of recombinant and synthetic antigens, developed using novel molecular techniques, have expanded diagnostic options as alternatives to native antigens directly isolated from cultivated parasites. For diagnosis of T. gondii infection in cats, sheep and pigs, some species-specific ELISAs are available that have performed well when compared with previous reference serological techniques, as reviewed by Wyrosdick and Schaefer (Reference Wyrosdick and Schaefer2015).
ELISAs for N. caninum antibodies in sera from dogs and cattle have been developed in several studies (Table 3). Conventional ELISAs using crude soluble antigen showed higher levels of serologic cross-reactivity to T. gondii when compared with IFAT (Björkman et al. Reference Björkman, Lunden, Holmdahl, Barber, Trees and Uggla1994; Silva et al. Reference Silva, Lobato, Mineo and Mineo2007). Serologic cross-reactivity with T. gondii was also observed when polyclonal mouse and cat sera were tested by a N. caninum ELISA using crude antigen (Nishikawa et al. Reference Nishikawa, Claveria, Fujisaki and Nagasawa2002). In contrast, the same mouse and cat sera did not cross-react by an ELISA based on N. caninum recombinant antigen (NcSRS2) (Nishikawa et al. Reference Nishikawa, Claveria, Fujisaki and Nagasawa2002). ELISAs prepared with N. caninum tachyzoite antigen associated with immunostimulating complexes (ISCOM), and using MAbs as secondary antibodies, presented better specificity than conventional ELISAs (Björkman et al. Reference Björkman, Lunden, Holmdahl, Barber, Trees and Uggla1994, Reference Björkman, Holmdahl and Uggla1997). The ISCOM particles have affinity for surface proteins, which minimizes interference by internal non-specific antigens (Björkman et al. Reference Björkman, Lunden, Holmdahl, Barber, Trees and Uggla1994). Moreover, none of the MAbs developed against N. caninum ISCOM incorporated antigens (including also Ncmab-4, and Ncmab-10 mentioned above) cross-reacted with T. gondii (Björkman and Lunden, Reference Björkman and Lunden1998). An ELISA based on immuno-affinity-purified native NcSRS2 showed no significant cross-reactions when tested with sera from cattle experimentally infected with a variety of protozoan parasites including also ten cattle infected with T. gondii (Schares et al. Reference Schares, Rauser, Sondgen, Rehberg, Barwald, Dubey, Edelhofer and Conraths2000). In addition, the TgSAG2A molecule has been demonstrated to be specific to T. gondii, considering that no cross-reactivity has been shown with N. caninum when using recombinant protein or even mimotopes derived from this molecular marker, as characterized by A4D12 MAb (Bela et al. Reference Bela, Oliveira Silva, Cunha-Junior, Pirovani, Chaves-Borges, Reis de Carvalho, Carrijo de Oliveira and Mineo2008; Carvalho et al. Reference Carvalho, Silva, Cunha-Junior, Souza, Oliveira, Bela, Faria, Lopes and Mineo2008; Cunha-Junior et al. Reference Cunha-Junior, Silva, Silva, Souza, Souza, Prudencio, Pirovani, Cezar, Barbosa, Goulart and Mineo2010; Santana et al. Reference Santana, Silva, Vaz, Pirovani, Barros, Lemos, Dietze, Mineo and Cunha-Junior2012; Macedo et al. Reference Macedo, Cunha, Cardoso, Silva, Santiago, Silva, Pirovani, Silva, Mineo and Mineo2013).
Table 3. Cross-reactions tested for Neospora caninum in published in-house ELISAs
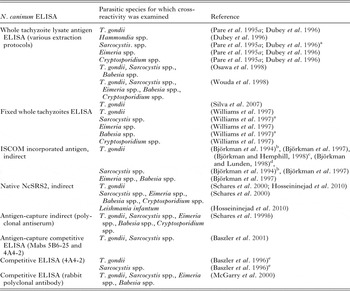
a Cross-reactions observed in serum dilutions lower than the cutoff.
b Rabbit sera against T. gondii and S. cruzi do not react in WB with ISCOM antigen.
c MAbs against ISCOM antigens do not recognize T. gondii in WB.
d Mouse serum immunized with ISCOM antigen does not recognize T. gondii.
e Some sera showed elevated levels of cross-reactions in WB.
Nowadays it is becoming clear that there is a need to characterize new molecular markers that are species-specific for T. gondii and N. caninum for the development of new diagnostic tools (Zhang et al. Reference Zhang, Lee, Yu, Kawano, Huang, Liao, Kawase, Zhang, Zhou, Fujisaki, Nishikawa and Xuan2011; Regidor-Cerrillo et al. Reference Regidor-Cerrillo, Garcia-Lunar, Pastor-Fernandez, Alvarez-Garcia, Collantes-Fernandez, Gomez-Bautista and Ortega-Mora2015). In this context, the identification of cross-reactive and species-specific antigens between N. caninum and T. gondii tachyzoites is mandatory and the proteomics approach constitutes an appropriate strategy for this purpose (Zhang et al. Reference Zhang, Lee, Yu, Kawano, Huang, Liao, Kawase, Zhang, Zhou, Fujisaki, Nishikawa and Xuan2011). These authors demonstrated the usefulness of proteomics to immuno-screen for cross-reactive or species-specific antigens from both parasites. Moreover, they showed that there was significant homology in the antigenic proteome profiles between the two parasites (Zhang et al. Reference Zhang, Lee, Yu, Kawano, Huang, Liao, Kawase, Zhang, Zhou, Fujisaki, Nishikawa and Xuan2011). Taking together, these findings shed light on the process to design new diagnostic tools in order to avoid cross-reactivity between N. caninum and T. gondii diagnostic tests.
The characterization of cross-reactive antigens between T. gondii and N. caninum has been achieved in some studies. An NTPase identified in N. caninum tachyzoites was antigenically cross-reactive to the NTPases of T. gondii (Asai et al. Reference Asai, Howe, Nakajima, Nozaki, Takeuchi and Sibley1998). Protein disulphide isomerase (PDI), heat-shock protein 70 (HSP70) and ribosomal protein P1 (RP1), were identified as cross-reactive antigens between the two parasites even when using MAbs, due to the high degree of homology among these parasite components (Liao et al. Reference Liao, Xuan, Huang, Shirafuji, Fukumoto, Hirata, Suzuki and Fujisaki2005a ). Zhang et al. (Reference Zhang, Compaore, Lee, Liao, Zhang, Sugimoto, Fujisaki, Nishikawa and Xuan2007a ) demonstrated that antibodies raised against the apical membrane antigen 1 of T. gondii (TgAMA 1) also recognize recombinant NcAMA 1. The ribosomal phosphoprotein (P0) was shown to be a cross-reactive antigen between T. gondii and N. caninum (Zhang et al. Reference Zhang, Lee, Liao, Compaore, Zhang, Kawase, Fujisaki, Sugimoto, Nishikawa and Xuan2007b ); antibodies raised against rNcPO inhibited the growth of both T. gondii and N. caninum tachyzoites. A protease with 42 kDa was localized in the rhoptry of T. gondii by means of a MAb; this MAb also reacted to a 42 kDa protein in N. caninum, which was also localized in the rhoptry of this parasite (Ahn et al. Reference Ahn, Song, Son, Shin and Nam2001).
The genome of N. caninum (NC-Liverpool strain) was compared with the available genome of the ME-49 strain of T. gondii (Reid et al. Reference Reid, Vermont, Cotton, Harris, Hill-Cawthorne, Konen-Waisman, Latham, Mourier, Norton, Quail, Sanders, Shanmugam, Sohal, Wasmuth, Brunk, Grigg, Howard, Parkinson, Roos, Trees, Berriman, Pain and Wastling2012). The authors found a high synteny between the two genomes and pointed out that most divergences occurred within the SRS antigens. Transcriptome analysis suggested that N. caninum uses fewer SRS antigens than T. gondii (Reid et al. Reference Reid, Vermont, Cotton, Harris, Hill-Cawthorne, Konen-Waisman, Latham, Mourier, Norton, Quail, Sanders, Shanmugam, Sohal, Wasmuth, Brunk, Grigg, Howard, Parkinson, Roos, Trees, Berriman, Pain and Wastling2012). Therefore, selecting those species-specific parasitic surface antigens for the establishment of serologic tests, such as SRSs, may favour the specificity of these tests.
Serological tests for N. caninum and cross-reactivity with Sarcocystis spp., B. besnoiti, N. hughesi and Hammondia spp.
Sera from Sarcocystis spp.-infected cattle have been shown to cross-react with several N. caninum antigens by WB (Baszler et al. Reference Baszler, Knowles, Dubey, Gay, Mathison and McElwain1996). However, antibodies against Sarcocystis spp. did not cross-react with N. caninum-immunodominant antigens (19, 29, 30 and 37 kDa) (Bjerkas et al. Reference Bjerkas, Jenkins and Dubey1994).
Sera from calves that were experimentally infected with Sarcocystis spp. tested positive by a conventional ELISA using crude N. caninum antigen (Dubey et al. Reference Dubey, Lindsay, Adams, Gay, Baszler, Blagburn and Thulliez1996); the same sera tested negative by N. caninum IFAT. In contrast, positive sera against several Sarcocystis spp. (S. cruzi, S. hirsuta, S. hominis and S. neurona) did not result in positive reactions in ELISAs based on N. caninum-selected antigens (ISCOM, whole-fixed tachyzoites, affinity-purified and recombinant) (Björkman et al. Reference Björkman, Lunden, Holmdahl, Barber, Trees and Uggla1994; Baszler et al. Reference Baszler, Knowles, Dubey, Gay, Mathison and McElwain1996, Reference Baszler, Adams, Vander-Schalie, Mathison and Kostovic2001; Lally et al. Reference Lally, Jenkins and Dubey1996; Schares et al. Reference Schares, Rauser, Sondgen, Rehberg, Barwald, Dubey, Edelhofer and Conraths2000; Howe et al. Reference Howe, Tang, Conrad, Sverlow, Dubey and Sibley2002). Serologic cross-reactivity between infections caused by N. caninum and Sarcocystis spp. seems to be neglegible when N. caninum-specific antigens are employed (Table 3).
Besnoitia besnoiti and N. caninum have cattle as their major hosts and may co-infect a high proportion of animals in regions where these parasites are endemic (Jacquiet et al. Reference Jacquiet, Lienard and Franc2010). Serologic tests such as indirect ELISA and IFAT had been developed over several decades for B. besnoiti antibodies (Frank et al. Reference Frank, Klinger and Pipano1970; Neuman, Reference Neuman1972; Janitschke et al. Reference Janitschke, De Vos and Bigalke1984; Shkap et al. Reference Shkap, Ungar-Waron, Pipano and Greenblatt1984), but at that time, the closely related parasite N. caninum was unknown, so the specificity of those tests could not have been ascertained. Later, it was shown that sera from N. caninum-positive cattle and gerbils recognized B. besnoiti antigens by IFAT when a less stringent cutoff (1:64) was used (Shkap et al. Reference Shkap, Reske, Pipano, Fish and Baszler2002); the authors also observed that these animal sera reacted against two bands of B. besnoiti antigens under reducing conditions by WB. In another study, a more stringent cutoff (1:200) for B. besnoiti IFAT did not show cross-reactions with sera from N. caninum-infected animals, whereas serum dilutions of 1:100 showed some level of cross-reactivity (Schares et al. Reference Schares, Basso, Majzoub, Rostaher, Scharr, Langenmayer, Selmair, Dubey, Cortes, Conraths and Gollnick2010).
Besnoitia besnoiti WBs and indirect ELISAs were developed for detection of antibodies in cattle and were tested for cross-reactivity with sera from animals infected with N. caninum and T. gondii (Cortes et al. Reference Cortes, Nunes, Reis, Staubli, Vidal, Sager, Leitao and Gottstein2006; Fernandez-Garcia et al. Reference Fernandez-Garcia, Alvarez-Garcia, Risco-Castillo, Aguado-Martinez, Marcen, Rojo-Montejo, Castillo and Ortega-Mora2010; Schares et al. Reference Schares, Basso, Majzoub, Rostaher, Scharr, Langenmayer, Selmair, Dubey, Cortes, Conraths, Haupt, Purro, Raeber, Buholzer and Gollnick2011). Cross-reactivity with N. caninum was observed in the tested ELISAs and WBs, especially with animals exhibiting high antibody titres for N. caninum. A novel ELISA for B. besnoiti was developed with affinity-purified antigens of confirmed relevance, mostly localized on the surface of tachyzoites. This ELISA has shown a lower degree of cross-reactivity with N. caninum (Schares et al. Reference Schares, Langenmayer, Scharr, Minke, Maksimov, Maksimov, Schares, Barwald, Basso, Dubey, Conraths and Gollnick2013). A recent study demonstrated that cattle exhibiting high antibody levels to N. caninum and/or Sarcocystis spp. presented a higher number of false-positive reactions for B. besnoiti by an in-house ELISA (Garcia-Lunar et al. Reference Garcia-Lunar, More, Campero, Ortega-Mora and Alvarez-Garcia2015); thus, infection with Sarcocystis spp. and N. caninum may augment cross-reactions with B. besnoiti.
No serologic cross-reactivity has been observed between N. caninum and H. heydorni. A few serum samples from mice, dog and sheep that were infected with H. heydorni did not cross-react with N. caninum antigens by IFAT, ELISA or WB (Nishikawa et al. Reference Nishikawa, Claveria, Fujisaki and Nagasawa2002; Gondim et al. Reference Gondim, Meyer, Peters, Rezende-Gondim, Vrhovec, Pantchev, Bauer, Conraths and Schares2015). However, further studies using larger numbers of sera from H. heydorni-infected animals are necessary to confirm the absence of cross-reactivity between N. caninum and H. heydorni.
Neospora hughesi was proposed as a new species based on seven nucleotide differences in the internal transcribed spacer 1 (ITS1) of the rDNA, as well as on ultrastructural and antigenic differences when compared with N. caninum (Marsh et al. Reference Marsh, Barr, Packham and Conrad1998). The acceptance of N. hughesi as a new species was reinforced by differences observed in amino acid sequences of two surface antigens (SAG1 and SRS2) to those from N. caninum (Marsh et al. Reference Marsh, Howe, Wang, Barr, Cannon and Conrad1999). However, polyclonal serum from a N. caninum-infected rabbit recognized NcSAG1, NhSAG1, NcSRS2 and NhSRS2 by immunoblot (Marsh et al. Reference Marsh, Howe, Wang, Barr, Cannon and Conrad1999). Differences also have been found in gene sequences between the dense granule proteins GRA6 and GRA7 of N. caninum and N. hughesi, although polyclonal serum to N. caninum recognized GRA6 and GRA7 antigens from both N. caninum and N. hughesi by immunoblot (Walsh et al. Reference Walsh, Vemulapalli, Sriranganathan, Zajac, Jenkins and Lindsay2001).
An ELISA for N. hughesi antibodies was developed using a recombinant NhSAG1 as antigen (Hoane et al. Reference Hoane, Yeargan, Stamper, Saville, Morrow, Lindsay and Howe2005); animals infected with N. hughesi presented a higher antibody reactivity to rNhSAG1 than to rNcSAG1, but the test was not able to unambiguously differentiate infections caused by N. hughesi or N. caninum. In another study, pre- and post-infection sera from dog and cattle that were experimentally inoculated with N. caninum were tested simultaneously by IFAT using tachyzoites of N. caninum or N. hughesi (Gondim et al. Reference Gondim, Lindsay and McAllister2009); all sera that tested positive for N. caninum also reacted with N. hughesi tachyzoites, although the antibody titres for N. hughesi IFAT were slightly lower as compared with the IFAT for N. caninum. To date, infections caused by N. caninum and N. hughesi cannot be serologically discriminated. As horses may be infected by both N. caninum and N. hughesi (Marsh et al. Reference Marsh, Howe, Wang, Barr, Cannon and Conrad1999; Pitel et al. Reference Pitel, Romand, Pronost, Foucher, Gargala, Maillard, Thulliez, Collobert-Laugier, Tainturier, Fortier and Ballet2003; Veronesi et al. Reference Veronesi, Diaferia, Mandara, Marenzoni, Cittadini and Piergili Fioretti2008), a species-specific serologic test for equines is desired.
Serological tests based on chimeric antigens, synthetic peptides and stage-specific antigens
Chimeric antigens
Almost 30 years ago, the production of recombinant polypeptides derived from genes encoding T. gondii antigens revolutionized the search for more efficient serologic methods (Johnson et al. Reference Johnson, Illana, McDonald and Asai1989; Johnson and Illana, Reference Johnson and Illana1991). The development of ELISAs based on a mixture of recombinant antigens, rather than the use of a single recombinant protein, has been presumed to increase the sensitivity of the ELISA for human sera, while maintaining the desired specificity (Johnson et al. Reference Johnson, Roberts and Tenter1992; Aubert et al. Reference Aubert, Maine, Villena, Hunt, Howard, Sheu, Brojanac, Chovan, Nowlan and Pinon2000). ELISAs containing a mixture of recombinant antigens were also tested for antibodies against T. gondii in sheep and cats (Tenter et al. Reference Tenter, Vietmeyer and Johnson1992).
A chimeric antigen is the fusion of gene fragments constructed as a single gene and expressed to form a hybrid protein (Yang et al. Reference Yang, Chang and Chao2004). The use of chimeric antigens for T. gondii serology is promising. Chimeric proteins are usually larger than single recombinant antigens resulting in a better binding to microtitre plates. In addition, as a chimeric antigen preparation consists of a single antigen, it may be easier to standardize than mixtures of recombinant antigens (Beghetto et al. Reference Beghetto, Spadoni, Bruno, Buffolano and Gargano2006; Lau et al. Reference Lau, Thiruvengadam, Lee and Fong2011; Holec-Gasior et al. Reference Holec-Gasior, Ferra and Drapala2012a ).
Several chimeric antigens have been tested by WB or ELISA for the detection of human antibodies against T. gondii. Among the developed chimeric antigens tested by serology, are preparations including parts of well-characterized immunodominant proteins, such as SAG1, SAG2, MIC1, MIC2, MIC3, MAG1, M2AP, GRA1, GRA2, GRA3 and ROP1 (Beghetto et al. Reference Beghetto, Spadoni, Bruno, Buffolano and Gargano2006; Lau et al. Reference Lau, Thiruvengadam, Lee and Fong2011; Holec-Gasior et al. Reference Holec-Gasior, Ferra and Drapala2012a , Reference Holec-Gasior, Ferra, Drapala, Lautenbach and Kur b ; Ferra et al. Reference Ferra, Holec-Gasior and Kur2015a ). The standardization of a chimeric-antigen-based test for T. gondii antibodies depends on various factors, including an optimal selection of antigens and proper expression of all desired epitopes. In addition to humans, a recent paper reports the first trial of chimeric antigens employed for T. gondii serology in farm animals (horses, swine and sheep) (Ferra et al. Reference Ferra, Holec-Gasior and Kur2015b ); the authors validated their antigen with more than 400 sera and also included 15 sera, which were serologically positive for N. caninum but negative for T. gondii. It is interesting to note that each animal species responded differently to the chimeric-antigen preparations used in the ELISAs, but the SAG2–GRA1–ROP1L construct reached the best overall specificity and sensitivity for the three tested species (Ferra et al. Reference Ferra, Holec-Gasior and Kur2015b ). Despite progress in the development of chimeric antigens for T. gondii serology, in particular for humans, inadequate information is available about the serologic cross-reactivity potential of those tests with sera from animals infected with other Toxoplasmatinae parasites.
Synthetic peptides, serotyping and stage-specific antigens
The combination of molecular engineering and chemical synthesis of antigens has been applied for the development of serological techniques with improved sensitivity and specificity. Synthetic peptides representing several epitopes of numerous antigens have been used in microarray assays for serotyping of viral and bacterial diseases (Neuman de Vegvar et al. Reference Neuman de Vegvar, Amara, Steinman, Utz, Robinson and Robinson2003; Nahtman et al. Reference Nahtman, Jernberg, Mahdavifar, Zerweck, Schutkowski, Maeurer and Reilly2007).
In T. gondii infections, the humoral response has been demonstrated to be partially strain-specific. In one study, MAbs produced against SAG2A from naturally infected mice recognized the surface antigens encoded by the SAG2 allele of type I and III strains, but not of type II strains (Parmley et al. Reference Parmley, Gross, Sucharczuk, Windeck, Sgarlato and Remington1994). Another study identified a MAb showing differences in the recognition of type II and III strains (Bohne et al. Reference Bohne, Gross and Heesemann1993). Kong et al. (Reference Kong, Grigg, Uyetake, Parmley and Boothroyd2003) screened nucleotide sequences from types I, II and III of T. gondii for the identification of polymorphic regions from genes coding selected antigens. Allele-specific peptides were synthetized and screened by ELISA using sera from mice and humans. Synthetic peptides based on SAG2A, GRA3, GRA6 and GRA7 were able to discriminate type II from non-type II infections (Kong et al. Reference Kong, Grigg, Uyetake, Parmley and Boothroyd2003). In subsequent studies, serotyping for T. gondii infections using new recombinant polypeptides or new or improved synthetic peptides, as well as target populations from different regions, have been performed by ELISAs (Peyron et al. Reference Peyron, Lobry, Musset, Ferrandiz, Gomez-Marin, Petersen, Meroni, Rausher, Mercier, Picot and Cesbron-Delauw2006; Sousa et al. Reference Sousa, Ajzenberg, Vilanova, Costa and Darde2008, Reference Sousa, Ajzenberg, Marle, Aubert, Villena, da Costa and Darde2009). In two studies, peptide-microarrays were validated to discriminate the serological responses against clonal-type T. gondii strains in samples from humans (Maksimov et al. Reference Maksimov, Zerweck, Maksimov, Hotop, Gross, Spekker, Daubener, Werdermann, Niederstrasser, Petri, Mertens, Ulrich, Conraths and Schares2012b ) and cats (Maksimov et al. Reference Maksimov, Zerweck, Dubey, Pantchev, Frey, Maksimov, Reimer, Schutkowski, Hosseininejad, Ziller, Conraths and Schares2013). The latter study used sera from experimentally infected cats for validation and showed significant type-specific differences in the IgG response against the tested peptide panel. However, in many peptides, reactions were not clonal type-specific (Maksimov et al. Reference Maksimov, Zerweck, Dubey, Pantchev, Frey, Maksimov, Reimer, Schutkowski, Hosseininejad, Ziller, Conraths and Schares2013).
A peptide microarray was developed and validated for serotyping T. gondii infections in humans, aiming to differentiate between different manifestations of T. gondii infection (Maksimov et al. Reference Maksimov, Zerweck, Maksimov, Hotop, Gross, Pleyer, Spekker, Daubener, Werdermann, Niederstrasser, Petri, Mertens, Ulrich, Conraths and Schares2012a ). Thirty eight T. gondii synthetic peptides, consisting of 18 peptides characterized in previous studies, and 20 novel peptides, predicted by bioinformatics approach, were tested to differentiate acute, latent and ocular infections. Some peptides based on dense granule and microneme antigens (GRA2-28, MIC3-282 and MIC3-191) showed promising results for differentiation between acute and latent infections (Maksimov et al. Reference Maksimov, Zerweck, Maksimov, Hotop, Gross, Pleyer, Spekker, Daubener, Werdermann, Niederstrasser, Petri, Mertens, Ulrich, Conraths and Schares2012a ).
The use of synthetic peptides for T. gondii serotyping may have a great potential for discriminating between T. gondii infections and infections caused by other parasite species expected to induce some degree of serologic cross-reactivity. Of course, one important prerequisite is that peptides applied in such tests are not specific for particular clonal-types or genotypes of T. gondii or related parasite species. In addition, potential cross-reactivities need to be addressed; for instance, synthetic peptides considered to react specifically with antibodies generated by T. gondii infections need to be tested with sera from animals exposed to H. hammondi, as this parasite has a very close genetic relationship to the former (Walzer et al. Reference Walzer, Adomako-Ankomah, Dam, Herrmann, Schares, Dubey and Boyle2013, Reference Walzer, Wier, Dam, Srinivasan, Borges, English, Herrmann, Schares, Dubey and Boyle2014). The use of T. gondii-specific peptides for diagnosis in animals should also be tested with N. caninum, which may cross-react, as shown in Table 3. To our knowledge, there are no published studies using synthetic peptides to differentiate infections in animals cause by T. gondii, H. hammondi or N. caninum.
Stage-specific antigens (native or recombinant) from T. gondii have been investigated for decades by several research groups, including oocysts and sporozoites which can be produced in cats (Kasper et al. Reference Kasper, Bradley and Pfefferkorn1984; Ferguson et al. Reference Ferguson, Brecht and Soldati2000; Dumetre and Darde, Reference Dumetre and Darde2007; Possenti et al. Reference Possenti, Cherchi, Bertuccini, Pozio, Dubey and Spano2010, Reference Possenti, Fratini, Fantozzi, Pozio, Dubey, Ponzi, Pizzi and Spano2013; Bushkin et al. Reference Bushkin, Motari, Magnelli, Gubbels, Dubey, Miska, Bullitt, Costello, Robbins and Samuelson2012; Fritz et al. Reference Fritz, Bowyer, Bogyo, Conrad and Boothroyd2012). In contrast, antigens from Neospora spp. are mostly obtained from tachyzoites. Only small numbers of studies have produced N. caninum tissue cysts in cell culture or in animals (Weiss et al. Reference Weiss, Ma, Halonen, McAllister and Zhang1999; Tunev et al. Reference Tunev, McAlilster, Anderson-Sprecher and Weiss2002; Risco-Castillo et al. Reference Risco-Castillo, Fernandez-Garcia and Ortega-Mora2004; Vonlaufen et al. Reference Vonlaufen, Guetg, Naguleswaran, Muller, Björkman, Schares, von Blumroeder, Ellis and Hemphill2004), or have induced oocyst production in canid definitive hosts (McAllister et al. Reference McAllister, Dubey, Lindsay, Jolley, Wills and McGuire1998; Schares et al. Reference Schares, Heydorn, Cuppers, Conraths and Mehlhorn2001; Gondim et al. Reference Gondim, Gao and McAllister2002).
The identification of the first bradyzoite-specific gene of N. caninum (NcSAG4), an orthologue to TgSAG4, allowed the production and characterization of the recombinant protein rNcSAG4 (Fernandez-Garcia et al. Reference Fernandez-Garcia, Risco-Castillo, Zaballos, Alvarez-Garcia and Ortega-Mora2006). This antigen has been applied for the development of a stage-specific ELISA for bovine neosporosis (Aguado-Martinez et al. Reference Aguado-Martinez, Alvarez-Garcia, Fernandez-Garcia, Risco-Castillo, Arnaiz-Seco, Rebordosa-Trigueros, Navarro-Lozano and Ortega-Mora2008).
The production of Hammondia spp. antigens is even more difficult, because there is no permanent culture for these parasites (Riahi et al. Reference Riahi, Darde, Bouteille, Leboutet and Pestre-Alexandre1995; Schares et al. Reference Schares, Meyer, Barwald, Conraths, Riebe, Bohne, Rohn and Peters2003; Gondim et al. Reference Gondim, Meyer, Peters, Rezende-Gondim, Vrhovec, Pantchev, Bauer, Conraths and Schares2015). Therefore, most antigens from Hammondia spp. are derived from oocysts or from parasite cysts from intermediate hosts (Riahi et al. Reference Riahi, Bouteille and Darde1998, Reference Riahi, Leboutet, Bouteille, Dubremetz and Darde1999, Reference Riahi, Leboutet, Labrousse, Bouteille and Darde2000; Walzer et al. Reference Walzer, Adomako-Ankomah, Dam, Herrmann, Schares, Dubey and Boyle2013). Generation of recombinant antigens from Hammondia spp. is lacking. It would enable studies on exposure of animals and humans to these parasites, as well as on serologic cross-reactivity with related protozoa.
A serologic test for humans based on a T. gondii sporozoite-specific protein has been evaluated and seems to represent a promising method to discriminate between oocyst-induced infections from those transmitted by ingestion of infected meat (Hill et al. Reference Hill, Coss, Dubey, Wroblewski, Sautter, Hosten, Munoz-Zanzi, Mui, Withers, Boyer, Hermes, Coyne, Jagdis, Burnett, McLeod, Morton, Robinson and McLeod2011), which is an important consideration in epidemiological investigations. This study was the first to use a sporozoite protein, called T. gondii embryogenesis-related protein (TgERP) in a serologic test for T. gondii. In a recent study another sporozoite-derived protein called CCp5A was successfully used in an ELISA to identify oocyst-infected animals (Santana et al. Reference Santana, Gebrim, Carvalho, Barros, Barros, Pajuaba, Messina, Possenti, Cherchi, Reiche, Navarro, Garcia, Pozio, Mineo, Spano and Mineo2015); this test was able to identify antibodies in sera from humans, pigs, mice and chickens that were naturally or experimentally infected with T. gondii oocysts and discriminate between animals infected from ingestion of oocysts or by carnivorism. Further investigations are necessary for validation and to confirm whether the sporozoite proteins TgERP (Hill et al. Reference Hill, Coss, Dubey, Wroblewski, Sautter, Hosten, Munoz-Zanzi, Mui, Withers, Boyer, Hermes, Coyne, Jagdis, Burnett, McLeod, Morton, Robinson and McLeod2011) and CCp5A (Santana et al. Reference Santana, Gebrim, Carvalho, Barros, Barros, Pajuaba, Messina, Possenti, Cherchi, Reiche, Navarro, Garcia, Pozio, Mineo, Spano and Mineo2015) cross-react with sporozoite proteins derived from Hammondia spp. and Neospora spp. It is also important to test whether low infectious dose with oocysts will elicit detectable antibodies to TgERP or CCp5A.
Type or stage-specific antigens have a wide spectrum of applications, including investigations of infection outbreaks and identification of risk factors in selected populations (e.g. ingestion of oocyst or tissue cysts). Those antigens should be used with caution and only after rigorous validation in epidemiological studies.
Concluding remarks
Most currently available serologic tests for T. gondii may show some level of cross-reactivity with related coccidia, in particular with H. hammondi and N. caninum. Serological cross-reactivity between T. gondii and N. caninum has been observed when crude antigen ELISAs are employed. Several ELISAs based on recombinant species-specific antigens did not present cross-reactivity with N. caninum. Therefore, T. gondii shares more surface antigens with H. hammondi than with N. caninum.
When detecting T. gondii antibodies in animals, IFAT seems to be more specific than the ELISAs based on crude antigen. Since N. caninum is not considered to be a human pathogen, the major concern regarding serologic cross-reactivity would be potential exposure to H. hammondi, although it is unknown whether this parasite is able to induce infection in humans.
Antibodies from cattle infected with B. besnoiti cross-react with N. caninum antigens by IFAT in serum dilutions lower than the recommended cutoff (1:200). Novel B. besnoiti ELISAs (e.g. ELISA based on purified surface antigens) showed a lower degree of cross-reactivity with sera from cattle infected with N. caninum or other related parasites. Antibodies against bovine Sarcocystis sp. present negligible cross-reactions with N. caninum-immunodominant antigens. The development of ELISAs for N. caninum antibodies based on chimeric peptides specific for the parasite seems to be promising. Chimeric antigens would enable a better standardization of serologic tests, as a single protein is used. Moreover, chimeric antigens would potentially present higher sensitivity than single recombinant antigens. Despite the close phylogenetic relationship between N. caninum and H. heydorni, no evidence of serologic cross-reactivity between these protozoa has been confirmed to date. Toxoplasma gondii infections cause more confusion with N. caninum serology than those infections induced by H. heydorni. Neospora caninum and N. hughesi infections cannot be serologically differentiated, because currently available serologic tests are genus rather than species-specific for these protozoa.
FINANCIAL SUPPORT
Luís Gondim was funded by Universidade Federal da Bahia (Brazilian Government) for the open access publication fees. José Mineo and Luís Gondim are recipients of scholarships from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazil.






