INTRODUCTION
Certain parasite species are known to induce changes to host behaviour as a means of increased trophic transmission, a process that represents one of the seminal examples of parasite-induced behavioural manipulation (Pearson, Reference Pearson1972; Bethel and Holmes, Reference Bethel and Holmes1973; Camp and Huizinga, Reference Camp and Huizinga1979; Helluy, Reference Helluy1983a , Reference Helluy b ; Combes, Reference Combes1991; Combes et al. Reference Combes, Fournier, Mone and Theron1994; Haas, Reference Haas1994; Esch et al. Reference Esch, Barger and Fellis2002; Mouritsen and Poulin, Reference Mouritsen and Poulin2002). Among intertidal animals, trematodes are one of the most common metazoan parasites (Mouritsen and Poulin, Reference Mouritsen and Poulin2002) and consist of an estimated 25 000 species, many of which are yet to be described (Esch et al. Reference Esch, Barger and Fellis2002). Trematodes are obligate parasites that have complex life cycles, infecting up to four hosts, taking many distinct forms and infecting their hosts in a variety of ways (Esch et al. Reference Esch, Barger and Fellis2002); with amphipod crustaceans generally often acting as second intermediate hosts (Pearson, Reference Pearson1972). It is known that for some trematode species, the metacercarial stage is capable of manipulating its intermediate host's behaviour to increase its chance of trophic transmission to the definitive host (Helluy, Reference Helluy1983a , Reference Helluy b ; Combes et al. Reference Combes, Fournier, Mone and Theron1994; Haas, Reference Haas1994; Esch et al. Reference Esch, Barger and Fellis2002; Mouritsen and Poulin, Reference Mouritsen and Poulin2002).
The widespread marine amphipod Echinogammarus marinus has been used to understand strategies employed by a diverse range of parasites capable of manipulating the reproductive biology of its host (Short et al. Reference Short, Guler, Yang, Kille and Ford2012). However, the discovery that E. marinus can also be infected with a parasite apparently capable of manipulating host behaviour (Guler and Ford, Reference Guler and Ford2010) has never been further investigated. In this case, infected specimens of the normally evasive E. marinus were found to spend significantly more time in the light and higher in the water column than uninfected individuals, a behaviour consistent with a parasitic strategy to increase trophic transmission. Such manipulative parasites are not unknown in amphipods, as the behaviour of infected E. marinus is similar to that observed in Gammarus insensibilis infected by the trematode Microphallus papillorobustus (Helluy, Reference Helluy1983a , Reference Helluy b ). It has been suggested that such parasites alter host behaviour by influencing pathways in a similar manner to neuropharmcological methods. For example, it appears that biogenic amines are frequently targeted by behaviour manipulating parasites (Nolan, Reference Nolan1998; Pryon and Elizee, Reference Pryon and Elizee2000; Klein, Reference Klein2003; Adamo, Reference Adamo2013; Helluy, Reference Helluy2013). Furthermore, components of the serotonin pathway have been implicated by experiments demonstrating that injection of serotonin, a monoamine neurotransmitter, into gammarids causesbehaviours similar to that induced by manipulating parasites (Helluy and Holmes, Reference Helluy and Holmes1990; Tain et al. Reference Tain, Perrot-Minnot and Cezilly2006; Perrot-Minnot et al. Reference Perrot-Minnot, Sanchez-Thirion and Cézilly2014), while other neurotransmitters at similar concentrations, failed to induce a comparable response (Helluy and Holmes, Reference Helluy and Holmes1990). In addition, it has been shown that once M. papillorobustus metacercariae encyst in the cerebral ganglia, specifically the protocerebrum responsible for all visual sensory input (Thomas et al. Reference Thomas, Guegan, Michalakis and Renaud2000; Helluy and Thomas, Reference Helluy and Thomas2003; Kostadinova and Mavrodieva, Reference Kostadinova and Mavrodieva2005), serotonin levels become altered in specific regions, with a decrease of 62% observed in the optic neuropils (Ponton et al. Reference Ponton, Lefevre, Lebarbenchon, Thomas, Loxdale, Marche, Renault, Perrot-Minnot and Biron2006), a decrease thought to be due to the degeneration of discrete sets of serotonergic neurons. Consistent with studies implicating serotonin related pathways, positive phototaxic and negative geotaxic behavioural changes have been observed in E. marinus exposed to serotonin and the selective serotonin reuptake inhibitor (SSRI) fluoxetine (Guler and Ford, Reference Guler and Ford2010). Uncovering the neurological pathways modulated by cerebral encysting behaviour-manipulating parasites promises to reveal profound insights into arthropod neurobiology (Poulin and Mouritsen, Reference Poulin and Mouritsen2006; Shaw et al. Reference Shaw, Korzan, Carpenter, Kuris, Lafferty, Summers and Overli2009; Helluy and Thomas, Reference Helluy and Thomas2010) and although our understanding of the molecular host–parasite interactions are still limited (Biron and Loxdale, Reference Biron and Loxdale2013), the existing investigations make it possible to credibly hypothesize which molecular pathways are altered in infected E. marinus. This opens the possibility of using the available E. marinus genomic resources (Short et al. Reference Short, Yang, Guler, Green Etxabe, Kille and Ford2014) to investigate the molecular biology underlying parasite-induced behavioural-manipulation at a transcriptomic level.
This study aims to identify the parasite species infecting E. marinus by comparing the parasite ribosomal RNA gene sequences with sequences deposited in public databases and those isolated from M. papillorobustus. Secondly, this study investigates the potential impact of the E. marinus parasite at the population level by comparing parasite prevalence with host abundance. Finally, we investigate the molecular pathways being altered in infected animals by attempting to identify expression changes in genes with plausible links to neurological pathways.
MATERIAL AND METHODS
Trematode harvesting and DNA isolation
Echinogammarus marinus were collected from beneath seaweed in the intertidal zone during low tide. Infected E. marinus individuals were taken from Langstone Harbour, Portsmouth, UK (50°47′23·13N 1°02′37·25W). Sixty adult males and females (n = 120) were collected during June and July 2011 and the location of the trematode metacercaria were recorded within the body cavity and head. Gammarus insensibilis infected by M. papillorobustus were collected from Étang de Thau, France (43°25′N, 3°35′E) and were kindly donated by Dr Frédéric Thomas (National Center of Scientific Research (CNRS) in Montpellier, France). Twenty cysts from each amphipod population were stored in 70% ethanol at −80 °C. DNA was extracted using the DNAeasy kit (Qiagen, UK) following the manufacturers’ protocol and quantified using a spectrophotometer (NanoDrop 1000).
Amplification of trematode ribosomal RNA genes
Primers were used to amplify the 18S (537F, 1133R, 1073F, 18SR, 18SF and 549R) (Near et al. Reference Near, Garey and Nadler1998) and 28S (LSU-5, 1500R) (Olson et al. Reference Olson, Cribb, Tkach, Bray and Littlewood2003) ribosomal RNA genes. Primers (PITSF, PITSR, Table 1) used to amplify the Internal Transcribed Spacer (ITS) region were designed using the 3′ end of the sequenced 18S region and the 5′ end of the 28S region. All primers were synthesized by Eurofins MWG Operon, Germany. Polymerase chain reaction (PCR) reactions were performed in 25 µL reactions containing 1·25 mm MgCl2, 1x PCR Buffer, 0·2 mm of each dNTP, 0·25 mm of each primer, 1 U Taq DNA polymerase (Promega, UK), 10 ng of genomic DNA. Reactions were carried out using the following thermal cycling conditions: 94 °C for 4 min followed by 32 cycles of 94 °C (45 s), 59 °C (45 s) and 72 °C (1·5 min), and a final incubation of 5 min at 72 °C. PCR products were analysed by agarose gel electrophoresis and were eluted and purified using the QIAquick Gel Extraction and purification Kit (Qiagen, UK) following the manufacturer's protocol. The purified PCR products were subsequently sequenced by Source Bioscience, UK.
Table 1. Primers designed using Primer-3 software [65] and synthesized by Eurofins MWG Operon

Phylogenetic analysis
The isolated parasite ribosomal sequences were used to perform a basic local alignment search tool (BLAST) analysis against the non-redundant sequences deposited in GenBank National Center for Biotechnology Information (NCBI, http://www.ncbi.nlm.nlh.gov) to reveal closely related sequences. The sequences obtained were aligned multiple sequence comparison by log-expectation (MUSCLE) and trimmed, before a phylogenetic tree was constructed using the maximum likelihood method implemented by the MEGA (v5·0) program (Hall, Reference Hall2013).
Population sampling
Echinogammarus marinus were collected from Langstone Harbour, Portsmouth, UK (50°47′23·13N 1°02′37·25W) during low tide by selecting five 1 m2 quadrats (total area = 5 m2) in the intertidal zone each month over an 18 month period (January 2010–June 2011). All algae and surface sediment (approximately 2 cm in depth) was retrieved and stored in polythene bags. In the laboratory, samples were washed and decanted through a 0·7 mm sieve and all algae were scraped to ensure all E. marinus individuals were collected. Individuals were separated into males, females and juveniles, and counted to assess relative abundances. To allow for later assessment of parasite prevalence and seasonality, 20 adults of each sex were selected from each monthly sample and stored in 70% ethanol at −80 °C.
PCR parasite screen
The DNA was isolated from the 20 adults of each sex set aside following the monthly sampling. The gonads and muscle tissue were dissected from the animal and washed with distilled water. DNA was extracted using the CHELEX© DNA extraction followed by a phenol-chloroform step and ethanol precipitation. Extracted DNA was quantified using a spectrophotometer (NanoDrop 1000). The samples were screened for evidence of infection using PCR. All PCR reactions were performed in 25 µL reactions containing 2·5 mm MgCl2, 0·25 mm of each dNTP, 0·5 mm of each primer (18SF 5-GATTAAGCCATGCATGCGTAAG-3 and Trem18SR1 5-GCCGCGGTAATTCCAGCTC-3), 1x PCR buffer, 1 U Taq DNA polymerase (Promega, UK) and 10 ng of template DNA. Reactions were carried out using the following thermal cycling conditions: 94 °C for 4 min followed by 32 cycles of 94 °C (45 s), 59 °C (45 s) and 72 °C (1·5 min), and a final incubation of 5 min at 72 °C. To check the quality of all DNA samples, amplification of the Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) gene was performed (Yang et al. Reference Yang, Short, Kille and Ford2011). PCR product size was visualized on a 1·2% agarose gel under a UV transilluminator following electrophoresis.
Statistical analysis
Relationships between two biological parameters, such as host abundance and parasite prevalence, were statistically analysed using Pearson's correlation coefficients. Relationships between parasite prevalence and environmental parameters were conducted using regression analysis performed with a statistical software package (SPSS® statistics v17·0·0).
Candidate gene selection and primer development
Genes with functional links to neurological and serotonin pathways in Drosophilia melanogaster were retrieved from FlyBase (flybase.org) and were used to perform a local BLAST search against the E. marinus transcriptome database (Short et al. Reference Short, Yang, Guler, Green Etxabe, Kille and Ford2014). Sequences sharing a high level of sequence identity (E-value ⩽ 1E-5) were taken and additional BLAST analyses (BLASTn) were performed against GenBank sequences (NCBI) to confirm the annotation. Gene candidates were selected on the basis of their direct involvement in the serotonin pathway or within processes that might plausibly influence behavioural responses in E. marinus (see Table 2). The only exception to this was a predicted Arginine Kinase, chosen due to its involvement in a specific immune response induced by the behaviour-manipulating trematode M. papillorobustus (Ponton et al. Reference Ponton, Lefevre, Lebarbenchon, Thomas, Loxdale, Marche, Renault, Perrot-Minnot and Biron2006). Primers were designed (Rozen and Skaletsky, Reference Rozen and Skaletsky2000) against the selected E. marinus contiguous sequence (Table 1) and synthesized by Eurofins MWG Operon, Germany. All primers were tested for their quantitative real-time PCR (qPCR) suitability using PCR carried out in 25 µL volume containing 1·5 mm MgCl2, 1x PCR buffer, 0·25 mm each dNTP, 1 U Taq DNA polymerase (Promega GoTaq®) and 10 ng cDNA (see section PCR parasite screen). A primer concentration 10 mm was determined to be suitable for all primers except ine1, 2 and 5-HT 1A , the concentrations were reduced to 5 mm to eliminate primer-dimer formation. Reactions were carried out using the following thermal cycling conditions: 95 °C (4 min) followed by 35 cycles of 95 °C (30 s), 60 °C (45 s), 72 °C (45 s) with a final incubation of 5 min at 72 °C. PCR products were analysed by agarose-gel electrophoresis to establish primer specificity.
Table 2. Identifying Echinogammarus marinus genes with putative neurological roles. Drosopholia genes with functional links to serotonin and neurological pathways were used to BLAST search the E. marinus transcriptome database to identify orthologous sequences. The retrieved E. marinus sequences were then compared with annotated sequences in GenBank (NCBI) database to confirm the putative annotation

RNA isolation and cDNA synthesis
The heads were removed from the bodies of infected and uninfected males (n = 18 in each group), before the first pereon and antennae were amputated. Heads were immediately snap frozen using liquid nitrogen following dissection and crushed in TRI Reagent® (Ambion, UK). The samples were divided to give three biological replicates for uninfected and infected groups (each replicate containing pooled head tissue from six animals). The total RNA was extracted using TRI Reagent® (Ambion, UK) according to manufacturer's instructions and cleaned using RNA Clean and Concentrator™−5 columns (Zymo Research, Orange, CA, USA). The RNA quantity and quality was assessed using a spectrophotometer (NanoDrop 1000) and agarose-gel electrophoresis, respectively. From the extracted RNA, 250 ng was reverse transcribed into cDNA using reverse transcriptase (Promega, UK) following the manufacturer's guidelines using OligoDT15 primers and RNasin Ribonuclease Inhibitor (NEB, UK). The quality of the resulting cDNA was tested by PCR amplification of the constitutively expressed GAPDH gene as previously described (Yang et al. Reference Yang, Short, Kille and Ford2011).
Q-PCR analysis
SYBR green-based qPCR was performed using a real-time PCR cycler (Eco Illumina). Each reaction was performed in triplicate and the absence of genomic DNA was confirmed by performing minus reverse transcription (RT) reactions for all samples. Reactions were carried out using GoTaq qPCR Master Mix (Promega, UK) in a 15 µL volume containing 1 µL of cDNA, 0·4 µL of the forward and reverse primer taken from a 10 µ m stock (note: 0·2 µL of forward and reverse primer was used for ine1, 2 and 5-HT 1A genes), 5·7 µL ultra-pure water and 7·5 µL of 2X GoTaq qPCR Master Mix. Reactions were carried out with Rox normalization and underwent an initial incubation step of 95 °C for 2 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 60 s, completed with 1 cycle of 60 °C for 95 s. Melt curve analysis was performed to confirm the specificity of the PCR product in each reaction. The amplification data were analysed by plotting the fluorescence signal ΔRn against the cycle number. An arbitrary threshold was selected within the linear phase of the log ΔRn against cycle number plot. The quantification cycle (Cq value) was determined to be the cycle number at which ΔRn crossed this threshold. A relative expression of a gene was determined using the ΔΔCq method, with normalization to the expression of the GAPDH reference gene, using the dedicated Eco (v3·0) software package. Statistical differences in the expression of infected vs unifected samples were compared using an independent t-test (α0·05).
RESULTS
Trematode characterization and identification
The parasite residing in E. marinus was found to encyst within the brain, hepatopancreas and abdomen, as well being attached to nerves in the thorax (Fig. 1). A survey of infection prevalence and intensity in 120 specimens during June and July 2011 revealed that approximately half the population were infected by one or more encysted trematodes (55·7% males and 50·7% females). The mean intensity (9·8) and abundance (4·9) of infection was greater for females than for males (4·8 and 2·7, respectively). Those that were found infecting the brain were found to make up approximately 7–9% of the total population (~20% of the infected portion) with similar infection between males and females The 18S, 28S and ITS regions of the parasite ribosomal RNA gene was obtained and used in conjunction with previously published sequences to perform a phylogenetic analysis. This reveals that the unknown parasite within the E. marinus population at Langstone Harbour is a trematode belonging to the family Microphallidae (Fig. 2). On the basis of rRNA gene sequence comparisons, the E. marinus parasite represents a new species of trematode falling between the branches of the genus Maritrema and Microphallus (Tkach et al. Reference Tkach, Littlewood, Olson, Kinsella and Swiderski2003).

Fig. 1. Metacercariae of trematodes that have encysted and melanized in the (a) hepatopancreas (scale bar = 200 µ m) and (b) ventral nerve cord of Echinogammarus marinus (scale bar = 100 µ m) and (c) unmelanized metacariae from E. marinus body cavity (scale bar = 400 µ m).
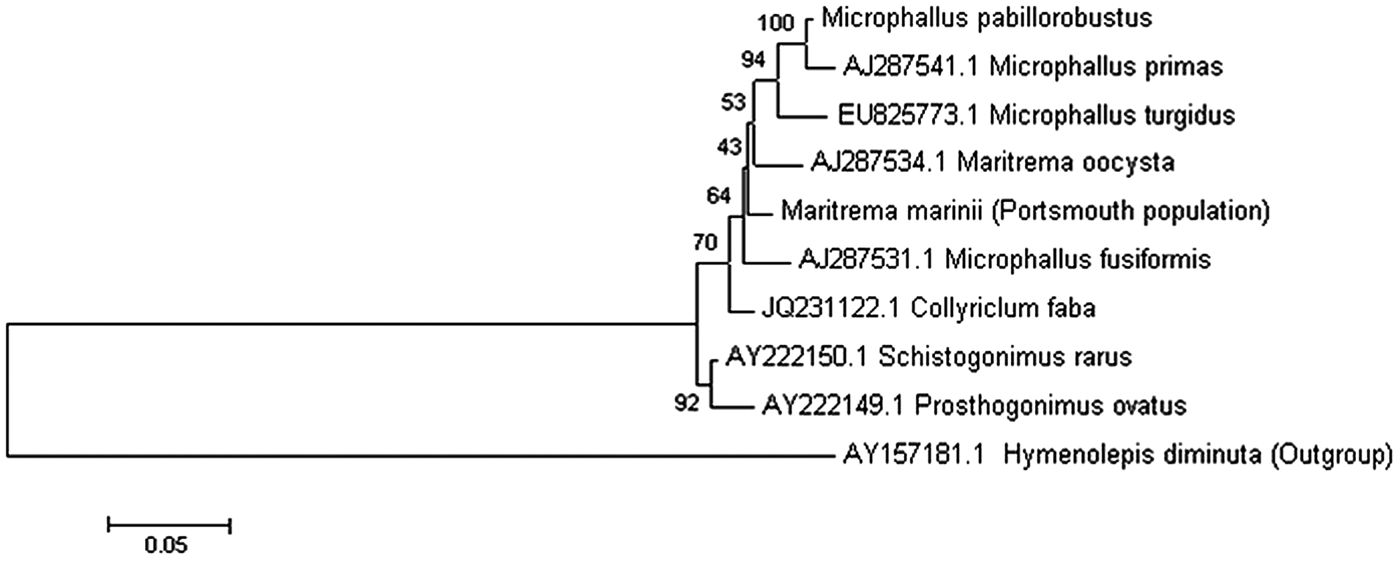
Fig. 2. Identification of unknown trematode species infecting Echinogammarus marinus at Langstone Harbour, UK. Molecular characterization of the well-studied trematode Microphallus pabillorobustus. A representative phylogenetic tree was generated using available rDNA sequences of digenean trematodes from NCBI. Sequences were aligned using MUSCLE and a phylogenetic tree was constructed using the maximum likelihood method implemented by the MEGA (Version 5) program. Bootstrap values (n = 100) for branches are shown as percentages. All branches are drawn to scale as indicated by the scale bar representing sequence divergence. The phylogenetic tree was generated as described above using trematode small subunit rDNA sequences (18S); scale bar represents 5% sequence divergence.
Determination of trematode seasonal prevalence
To assess whether the trematode infecting E. marinus may cause population level effects, an 18-month field study (January 2010–June 2011) was undertaken to determine if parasite prevalence significantly correlates with host abundance. The overall mean infection prevalence over the study period was 40·2 ± 3·6% and ranged from 17·5 to 70·0%. Infection prevalence peaked in February and July for both 2010 and 2011, with a crash in infection rates in October 2010 (Fig. 3). There was no correlation observed between the trematode prevalence and host abundance when directly comparing the months (Pearson's correlation coefficient; R = −0·330, P = 0·168). However, as trematode prevalence can have a delayed effect on host abundance (due to the time required to produce a development stage capable of definitive host infection), the trematode prevalence was aligned +1 month. This adjustment resulted in a significant negative relationship between host abundance and parasite prevalence (Pearson's correlation coefficient; R = −0·461, P = 0·047) (Fig. 4). Regression analysis failed to detect a relationship between trematode prevalence and environmental parameters such as sea temperature (P = 0·135, R = 0·582, df = 1, F = 0·315) or salinity (P = 0·282, R = 0·260, df = 1, F = 1·236).

Fig. 3. Overall monthly infections prevalence of trematode sp. infecting Echinogammarus marinus population at Langstone Harbour (Portsmouth, UK) between January 2010 and July 2011.

Fig. 4. Linear relationship between Echinogammarus marinus density from Langstone Harbour, Portsmouth (UK) and prevalence of a trematode parasite. Data obtained from field study during 2009–2011.
Gene expression analysis
Following candidate gene selection, ten sequences, representing genes with plausible links to neuronal pathways (Table 2), were chosen from the E. marinus transcriptome (Short et al. Reference Short, Yang, Guler, Green Etxabe, Kille and Ford2014) for expression analysis in infected and uninfected animals. Three biologically independent repeats indicated several genes present altered expression in infected animals (Fig. 5; Table 3). The expression of putative Tryptophan 5-monooxygenase activation protein gene (Ty3), two putative Rhodopsin genes (Rhod 1 and Rhod 2), the putative amino acid nutrient transporter gene (AT), the putative Arginine Kinase gene (AK) and the inebriated-like neurotransmitter gene 1 (Ine1) present no altered expression in trematode infected E. marinus. However, the putative serotonin receptor gene (5-HT 1A ) and tryptophan hydroxylase gene (PH) present consistently increased expression in infected animals in each biologically independent trial, with 5-HT 1A showing the highest overall mean fold change. In contrast, the inebriated-like neurotransmitter gene 2 (Ine2) and the putative amino acid decarboxylase gene (AD) present reduced expression in infected animals. For the two genes that present the greatest overall fold change in infected animals (5-HT 1A and Ine2), the greatest change in expression was observed in experimental repeat 1 (Fig. 5). Despite some consistency in the up or downregulation of genes, no significant differences were observed in the mean expression values between infected and uninfected samples (P > 0·05).

Fig. 5. Gene expression changes in Echinogammarus marinus induced by trematode infection in three independent trials with a mean and standard error (±1 s.e.). qPCR analysis of possible serotonin related genes using E. marinus head cDNA pooled (n = 6) control (uninfected) and infected trematode for each trial. Abbreviation: qPCR, quantitative real-time polymerase chain reaction. Bars represent mean fold change and error bars ±1 s.e.
Table 3. Fold change in expression of putative neurological genes in trematode infected normalized Echinogammarus marinus to uninfected individuals

AD, acid decarboxylase gene; AK, Arginine Kinase gene; AT, acid nutrient transporter gene; Ine1 and Ine 2, inebriated-like neurotransmitter gene 1 and 2; Ty3, tryptophan 5-monooxygenase activation protein gene; Rhod2 and Rhod1, two putative Rhodopsin genes; PH, tryptophan hydroxylase gene; 5HT1, 5-hydroxytryptamine.
DISCUSSION
Parasites account for a substantial portion of an ecosystem's total biomass (Kuris et al. Reference Kuris, Hechinger, Shaw, Whitney, Aguirre-Macedo, Boch, Dobson, Dunham, Fredensborg, Huspeni, Lorda, Mababa, Mancini, Mora, Pickering, Talhouk, Torchin and Lafferty2008) and play pivotal roles in community structures and ecosystem dynamics (Holmes, Reference Holmes1996; Horwitz and Wilcox, Reference Horwitz and Wilcox2005; Hudson et al. Reference Hudson, Dobson and Lafferty2006; Wood et al. Reference Wood, Byers, Cottingham, Altman, Donahue and Blakeslee2007; Lefevre et al. Reference Lefevre, Lebarbenchon, Gauthier-Clerc, Misse, Poulin and Thomas2009). Parasites that are capable of manipulating their host's behaviour to enhance transmission are of particular interest to both ecologists and parasitologists (Poulin and Mouritsen, Reference Poulin and Mouritsen2006; Lefevre et al. Reference Lefevre, Lebarbenchon, Gauthier-Clerc, Misse, Poulin and Thomas2009). This study investigates one such parasite infecting a population of the amphipod E. marinus. The rRNA gene subunit sequences revealed the parasite to be a trematode within the Microphallidae family. Our findings suggest this newly described species can dramatically influence its E. marinus host population. The parasite prevalence reached almost 70% at some points of the year and was found to significantly correlate with host abundance in a negative fashion. It is thought that populations of Corophium amphipods infected with trematodes are prone to local collapse due to the capacity of its parasites to induce behavioural change (Damsgaard et al. Reference Damsgaard, Mouritsen and Jensen2005), and our findings are certainly consistent with the hypothesis that trematodes can have a major impact on amphipod populations. In addition, we found no significant relationship between trematode abundance and any recorded environmental parameter, a finding also consistent with other trematode and host population studies (Lagrue and Poulin, Reference Lagrue and Poulin2008). The large fluctuations in the prevalence of trematode infection possibly reflect a build-up of individuals infected with trematode cysts undergoing a period of development prior to reaching the infective stage, after which the parasite manipulates its host in an attempt to complete its life cycle. If successful, this manipulative strategy would cause an increase in mortality biased towards infected individuals, leading to a reduced prevalence of infection within the population. This scenario is consistent with the observed infection fluctuations in E. marinus. Further investigation is needed to determine the extent to which mortality results from increased predation facilitated by behavioural manipulation or the pathogenic effects of infection. However, mortality as a result of increased predation is in congruence with laboratory experiments revealing this parasite has the capacity to significantly influence E. marinus behaviour (Guler and Ford, Reference Guler and Ford2010). In combination with other studies, our findings emphasize the potential importance of a parasite to the population dynamics of its host (Poulin and Mouritsen, Reference Poulin and Mouritsen2006).
This study attempts to link the altered E. marinus behaviour observed in infected individuals (Guler and Ford, Reference Guler and Ford2010) with the altered expression of genes with putative neurological functions. The majority of metacercariae were not found in the head region, but heavily encysted and melanized throughout the body cavity, including the hepatopancreas and encysting the ventral nerve cord. Whether this parasite can influence host behaviour from elsewhere in the hosts body (i.e. not within the head) is an interesting avenue to pursue especially given the production of serotonin throughout the ventral nerve cord (Harzsch and Waloszek, Reference Harzsch and Waloszek2000) The ‘neurological’ sequences retrieved from the E. marinus transcriptome (Short et al. Reference Short, Yang, Guler, Green Etxabe, Kille and Ford2014) were annotated by comparison with publicly available gene sequences possessing varying levels of functional annotation. The evolutionary divergence between E. marinus and other arthropod species with well-annotated genomes means the extent of functional overlap between orthologous genes is uncertain. However, the degree of sequence similarity suggests that, even if some functional divergence has occurred, the E. marinus sequences likely represent genes with closely related neurological roles.
Several putative neurological genes were consistently up or downregulated across the three biologically independent repeats (e.g. putative 5-HT 1A and Ine2, respectively) but with a notable extent of variation between the biological repeats. This variation may be due to several factors. Previously it has been shown that parasite load (Thomas and Poulin, Reference Thomas and Poulin1998), age and size of parasite (Benesh et al. Reference Benesh, Valtonen and Seppaelae2008), age of host (Poulin, Reference Poulin1993), infection by multiple parasite species (Cezilly et al. Reference Cezilly, Gregoire and Bertin2000; Haine et al. Reference Haine, Boucansaud and Rigaud2005) and seasonality (Brodeur and McNeil, Reference Brodeur and McNeil1989) can all affect the intensity of parasitic manipulation. In addition, these genes can be putatively linked to serotonin pathways. Serotonin is critical to many biological processes in invertebrates, including feeding, metabolism, moulting and reproduction (Yeoman et al. Reference Yeoman, Kemenes, Benjamin and Elliott1994; Fong, Reference Fong1998, Reference Fong, Daughton and Jones-Lepp2001), and the variation may reflect the involvement of these other processes. Overall, despite the variation, there is broad consistency in gene expression patterns between repeats, suggesting we have revealed the reliable alteration of ‘neurological’ gene expression associated with infection of M. marinnii. Of course, even assuming the chosen sequences represent genes critical to the regulation of host behaviour, it would still be uncertain whether the extent of altered regulation (e.g. ~4-fold in the case of 5-HT 1A ) is sufficient to induce the observed behavioural changes (Guler and Ford, Reference Guler and Ford2010). However, it is known that subtle differences in gene expression can lead to substantial neurological dysfunction (Tudor et al. Reference Tudor, Akbarian, Chen and Jaenisch2002) and therefore the observed expression changes in E. marinus may, to some extent, account for the observed behavioural phenotype.
A trend in the expression of several genes with putative links to the serotonin pathway was found in infected E. marinus. In all three repeats, the gene encoding a putative 5-HT 1A receptor gene and a tryptophan hydroxylase gene (PH) was upregulated in the infected groups. The 5-HT1A receptor is a transmembrane, G-protein coupled, somatodendritic autoreceptor within the dorsal raphe neurons and mediates inhibitory neurotransmission (Hall and Wedel, Reference Hall and Wedel1985). The activation of serotonin 1A receptors blocks subsequent serotonin release at the axon terminal, therefore significantly influences serotonin regulation in the brain (Riad et al. Reference Riad, Garcia, Watkins, Jodoin, Doucet, Langlois, El Mestikawy, Hamon and Descarries2000). Interestingly, the 5-HT1A receptor is believed to play a pivotal role in desensitization following chronic administration of SSRIs and restraints serotonin elevation (Hjorth et al. Reference Hjorth, Bengtsson, Kullberg, Carlzon, Peilot and Auerbach2000). This raises the hypothesis that the increased expression of this gene is an attempt by the host to counter balance the chronic elevation of serotonin induced by parasite infection. The 5-HT1A receptor represents just a single receptor 5-HT subtype. Therefore, it would be of interest, given that various subtypes play different roles in modulating serotonin levels (Hjorth et al. Reference Hjorth, Bengtsson, Kullberg, Carlzon, Peilot and Auerbach2000), to investigate the expression patterns of a larger range of receptor subtypes once a more complete E. marinus genomic resource is available. Such investigations have been attempted in the amphipod Gammarus pulex and suggest the 5-HT2 receptor plays an important role in photic behaviour. In addition, the same study linked the histaminergic system with behavioural modulation, revealing the molecular interactions may be more complex than originally believed and suggesting potential future directions of study (Perrot-Minnot et al. Reference Perrot-Minnot, Dion and Cézilly2013). Tryptophan hydroxylase (PH) is a rate-limiting enzyme that catalyses serotonin biosynthesis in the serotonergic nerves (Kim et al. Reference Kim, Park and Hwang2002). Therefore, the upregulation of this gene could potentially increase the biosynthesis of serotonin within the host brain. This hypothesis is supported by studies that have shown upregulation of a tryptophan hydroxylase gene in rats exposed to SSRIs (Kim et al. Reference Kim, Park and Hwang2002; Shishkina et al. Reference Shishkina, Kalinina and Dygalo2007). A recent study that measured gene expression in E. marinus exposed to the SSRIs sertraline and fluoxetine found no significant change in the expression of PH (Bossus et al. Reference Bossus, Guler, Short, Morrison and Ford2014), however, gene expression levels were measured following an 8 day exposure and infecting trematode parasites have considerably longer to modulate their hosts’ gene expression. Furthermore, we do not currently have a detailed mechanistic understanding of the gene pathways influenced, it is possible that SSRIs and manipulating parasites influence serotonin related pathways in different ways and therefore cause distinct gene expression profiles. Whether the upregulation of the 5-HT 1A gene expression is in response to elevated PH levels is unclear at this stage but is a plausible hypothesis.
A slight trend in downregulation of the putative amino acid decarboxylase (AD) was observed in all experimental repeats. This was unexpected, as past work on gammarids infected with behaviour manipulating parasites have demonstrated increased expression of aromatic L-amino acid decarboxylase proteins (Kostadinova and Mavrodieva, Reference Kostadinova and Mavrodieva2005). Although, it should also be noted that the differential expression of aromatic L-amino acid decarboxylase was shown in gammarid species that display only altered phototaxis (Kostadinova and Mavrodieva, Reference Kostadinova and Mavrodieva2005), rather than combined phototaxic and geotaxic behavioural responses (Guler and Ford, Reference Guler and Ford2010). However, as this study also finds upregulation of a putative tryptophan hydroxylase (PH), the downregulation, albeit small, of the AD gene is somewhat incongruous, as both enzymes are involved in serotonin synthesis, and requires further investigation.
A study investigating the proteomic response of amphipod hosts to infection by behaviour manipulating parasites revealed that Arginine Kinase is induced in infected animals (Ponton et al. Reference Ponton, Lefevre, Lebarbenchon, Thomas, Loxdale, Marche, Renault, Perrot-Minnot and Biron2006). However, our study found no altered expression of the putative Arginine Kinase gene in trematode infected E. marinus. This difference may reflect divergent molecular strategies employed by various parasite species or a differential response by different host species. Of course, as our study measured mRNA levels, it is also possible that Arginine Kinase is post-transcriptionally regulated, leading to changes in proteins levels in infected animals that are not reflected by changes to transcript abundance.
The putative E. marinus inebriated-like neurotransmitter gene 2 (Ine2) presented consistent trend in downregulation in infected groups. Ine2 is a neurotransmitter in Drosophila that resembles Na+/Cl−-dependent neurotransmitters, a family of transporters responsible for catalysing the rapid reuptake and release of neurotransmitters, such as serotonin, dopamine and norepinephrine, into the synapse (Soehnge et al. Reference Soehnge, Huang, Becker, Whitley, Conover and Stern1996). These neurotransmitters appear to play important roles in regulating behaviour. Dopamine deficiency in Drosophila can lead to several changes in behavioural traits including phototaxis, activity levels, negative geotaxis and olfactory learning (Riemensperger et al. Reference Riemensperger, Isabel, Coulom, Neuser, Seugnet, Kume, Iché-Torres, Cassar, Strauss and Preat2011). It has also been shown that inebriated mutants present defective reuptake of neurotransmitters causing over stimulation of motor neurons and oscillations of the light-induced photoreceptor potential (Huang et al. Reference Huang, Huang, Chinnappan, Bocchini, Gustin and Stern2002). The extent to which the Drosophila and putative E. marinus inebriated genes share a function is uncertain, however, given the sequence similarity, it is likely the putative E. marinus Ine2 represents a closely related neurotransmitter. Although considerable work is required to better understand the function of this gene in E. marinus, it is plausible that its downregulation could lead to elevated neurotransmitter levels that could conceivably be associated with the behavioural traits observed in infected individuals.
New technologies applied to the study of animal behaviour are revealing links between the underlying genome and behavioural phenotypes (Bell and Robinson, Reference Bell and Robinson2011). Such investigations will elucidate the genetic basis of novel behaviours and help answer important evolutionary questions about the life histories of parasites and their hosts. Furthermore, a better understanding of the pathways from genes to phenotypes will increase our knowledge of the mechanistic basis of animal behaviour. Amphipods clearly represent one of the best animal groups for the study of parasite-induced behavioural manipulation (Bethel and Holmes, Reference Bethel and Holmes1973; Helluy, Reference Helluy1983a , Reference Helluy b , Reference Helluy2013; Helluy and Holmes, Reference Helluy and Holmes1990; Cezilly et al. Reference Cezilly, Gregoire and Bertin2000; Helluy and Thomas, Reference Helluy and Thomas2003; Damsgaard et al. Reference Damsgaard, Mouritsen and Jensen2005; Kostadinova and Mavrodieva, Reference Kostadinova and Mavrodieva2005; Ponton et al. Reference Ponton, Biron, Joly, Helluy, Duneau and Thomas2005, Reference Ponton, Lefevre, Lebarbenchon, Thomas, Loxdale, Marche, Renault, Perrot-Minnot and Biron2006; Leung and Poulin, Reference Leung and Poulin2006; Tain et al. Reference Tain, Perrot-Minnot and Cézilly2007). However, in comparison with well-established model arthropod species, investigations using amphipods have been hampered by a lack of genomic resources and an inability to apply transgenic technologies. However, the progress currently being made in both areas (Rehm et al. Reference Rehm, Hannibal, Chaw, Vargas-Vila and Patel2009; Zeng et al. Reference Zeng, Villanueva, Ewen-Campen, Alwes, Browne and Extavour2011; Hook et al. Reference Hook, Twine, Simpson, Spadaro, Moncuquet and Wilkins2013; Christie, Reference Christie2014; Short et al. Reference Short, Yang, Guler, Green Etxabe, Kille and Ford2014) will greatly facilitate research uncovering the molecular biology underlying parasite-induced behavioural manipulation.
In summary, this study found the seasonal prevalence of a newly identified species of parasite infecting an amphipod population significantly correlated with host abundance in a negative fashion. When taken together with behavioural assays (Guler and Ford, Reference Guler and Ford2010) and the observed transcriptomic alterations, our findings are consistent with the hypothesis that this trematode species can alter the behaviour of its host by modulating neuronal processes, and this influence increases the likelihood of predation to the extent of causing population level effects. These findings add to the mounting evidence that parasites alter their host's behaviour in ways that promote transmission and represent some of the first links between parasite-induced behavioural manipulation of amphipods and changes in gene expression. Although the data we present here are clearly of a preliminary nature, this study has produced a foundation for a deeper understanding of the consequences of trematode infection the amphipod E. marinus.
ACKNOWLEDGEMENTS
We are very appreciative to Dr Frédéric Thomas for kindly donating G. insensibilis infected by M. pabillorobustus.
FINANCIAL SUPPORT
Y. G and S. S are supported by the Natural Environment Research Council (UK) grant (NE/G004587/1) awarded to P. K and A. T. F.











