Introduction
The burden of mental illness is a growing problem globally, with approximately 80% of people in low- to middle-income countries (LMIC) likely to experience a mental disorder within their lifetime (Wilson, Reference Wilson2016). Psychiatric disorders (also referred to as mental illnesses) are psychological and behavioural patterns or syndromes that cause significant distress, impairment of personal functioning, involving abnormal emotions, thoughts and behaviours (Parekh, Reference Parekh2018). Psychiatric disorders make up 30% of the global disease burden; however, only 3% of global health investment is directed towards them, which drops to 1% in Africa (Vigo et al., Reference Vigo, Thornicroft and Atun2016). Half of the individuals living with a mental illness in high-income countries receive insufficient care, and this figure rises to nearly 90% in low-income countries (WHO and DO, Reference WHO and DO2017). The standard of care for mental illnesses in low-income countries has declined further during the global coronavirus disease 2019 (COVID-19) pandemic due to breakdown or health services provision as well as the socioeconomic impacts of COVID-19 mitigation strategies such as lockdown (Sodi et al., Reference Sodi, Modipane, Oppong Asante, Quarshie, Asatsa, Mutambara and Khombo2021).
The aetiology and therefore treatment of mental illness is complex. Various systemic infections can lead to a syndrome known as sickness behaviour, which is a behavioural complex induced by infections and immune trauma, mediated by pro-inflammatory cytokines (Kelley and Kent, Reference Kelley and Kent2020). The term ‘sickness behaviour’ is theorized to be, in part, orchestrated by inflammatory responses, which can trigger changes including increased hypervigilance and decreased motivation. The hypothesis postulates that these behaviours are thought to be evolutionarily advantageous in aiding survival by allocating more energy to the healing process (Miller and Raison, Reference Miller and Raison2016).
Humans are host to nearly 300 species of parasitic worms and over 70 species of protozoa, with over a billion people affected by helminth infections, causing chronic inflammation (WHO, 2020). Many socioeconomic, emotional and psychological factors have long been associated with contributing to and facilitating depression. However, recent empirical work has found that biological factors, such as higher baseline inflammation due to high pathogen environments, as being potential contributors to the development of depression (Zunszain et al., Reference Zunszain, Hepgul and Pariante2012; Stieglitz et al., Reference Stieglitz, Trumble, Thompson, Blackwell, Kaplan and Gurven2015; Felger, Reference Felger, Macaluso and Preskorn2019; Lee and Giuliani, Reference Lee and Giuliani2019). A higher prevalence of psychiatric disorders in countries where parasite infections are more prevalent suggests that parasitic infections may play a role in psychiatric disorders. An increased parasitic load is associated with changes in mental health status; and the notion that treating psychiatric patients for chronic inflammation, caused by pathogens such as parasites, can improve mental health outcomes has gained momentum (Fond et al., Reference Fond, Hamdani, Kapczinski, Boukouaci, Drancourt, Dargel, Oliveira, Le Guen, Marlinge, Tamouza and Leboyer2014; Na et al., Reference Na, Lee, Lee, Cho and Jung2014; Pariante, Reference Pariante2016). It has been proposed that in addition to suppressing the immune system, pathogens also induce the release of neuromodulators that regulate mental states (Adamo, Reference Adamo2002), some of which appear to be induced by endogenous opiates (Kavaliers et al., Reference Kavaliers, Colwell and Choleris1999). For example, the parasite Schistosoma mansoni produces opioid peptides, which are thought to suppress the immune system (Pryor and Elizee, Reference Pryor and Elizee2000). However, whether parasite-produced opioids have a direct effect on the CNS and behaviour is unknown. It has been theorized that as opioids are both immune and neuromodulators, the parasite could potentially use the same compound to suppress the host's immune system and alter neural function (Salzet, Reference Salzet2000). Or, through more indirect means, the parasite could manipulate immune-neural connections in the host to alter neural functions and change behaviour (Thompson and Kavaliers, Reference Thompson and Kavaliers1994; Kavaliers et al., Reference Kavaliers, Colwell and Choleris1999). Genes coding for an increased anti-pathogen immune response have been selected for as they were beneficial to host survival, leading to the ‘inflammation bias’ in the human genome (Miller and Raison, Reference Miller and Raison2016). Whilst this inflammatory bias has aided mankind well, in protecting against pathogens and ensuring the survival and propagation of humans as a species, it is important to recognize that successful defence against pathogens is reliant on both immunologic and behavioural responses. Considering the intimate relationship existing between the brain and the immune system, it has been suggested that a consequence of the inflammatory bias is susceptibility to behavioural and mental disorders, including reduced exploratory behaviour manifesting in the form of depression and hypervigilance in the form of anxiety (Raison and Miller, Reference Raison and Miller2013). Supporting this notion is empirical work suggesting that depression risk alleles are regularly associated with immune responses to infection that were likely to enhance survival in ancestral environments (Raison et al., Reference Raison, Rutherford, Woolwine, Shuo, Schettler, Drake, Haroon and Miller2013; Raison and Miller, Reference Raison and Miller2013).
Depressive symptoms are consistently associated with inflammation in both human and animal models (van den Biggelaar et al., Reference van den Biggelaar, Gussekloo, de Craen, Frölich, Stek, van der Mast and Westendorp2007; Dantzer et al., Reference Dantzer, O'Connor, Freund, Johnson and Kelley2008). Risk alleles for depression are high in the general population and they are thought to have been retained to promote defence against pathogens through a range of immunological and behavioural responses (Raison and Miller, Reference Raison and Miller2017). The genes involved with such responses to pathogens have been shown to be altered in schizophrenic (Sainz et al., Reference Sainz, Mata, Barrera, Perez-Iglesias, Varela, Arranz, Rodriguez and Crespo-Facorro2013) and anxiety disorders (Luciano et al., Reference Luciano, Houlihan, Harris, Gow, Hayward, Starr and Deary2010). Furthermore, it has been observed in empirical work that inflammation in otherwise healthy individuals, can work as a potential predictor of future development of psychopathology over the subsequent months or years (Valkanova et al., Reference Valkanova, Ebmeier and Allan2013). In addition, reducing inflammation therapeutically improves conditions such as depression in some patients (Raison et al., Reference Raison, Rutherford, Woolwine, Shuo, Schettler, Drake, Haroon and Miller2013). Whilst such work does provide support to the hypothesis that a causational pathway exists between inflammation and some mental illnesses, more evidence is required to determine the exact nature of this association.
There are now concerted efforts to understand the mechanisms underlying the aetiology of mental illness. Nonetheless, these efforts are slow in relation to the increase of the burden of mental illness and the contribution of pathogens remains poorly understood as there are many other contributing and potentially confounding proximal pathways that blur the lines of pathogenic and inflammatory contributions to the development of mental illness. Over one billion people are affected by helminth infections and most of these are chronic infections causing chronic inflammation (WHO, 2020). During chronic inflammation, physiological processes become compromised through prolonged cytokine release. This can cause alterations to metabolism and homoeostatic set points. Inflammatory mediators such as tumour necrosis factor alpha (TNF-α), interleukin-6 (IL6) and prostaglandins are involved in hypothalamic–pituitary–adrenal (HPA) regulation. The HPA axis dysfunction is implicated in psychiatric conditions such as bipolar disorder and schizophrenia (Tsigos and Chrousos, Reference Tsigos and Chrousos1994; Pariante and Lightman, Reference Pariante and Lightman2008).
Africa carries the highest burden of infectious diseases, with a particularly large burden of helminth and protozoan infections (Orish, Reference Orish2015). To date, there has been no analysis of the association between parasitic infection and mental illness in Africa. This knowledge would inform prioritization and integrated health interventions within health systems often working with limited budgets. In this systematic review, the primary aim was to investigate the prevalence of mental illness associated with parasitic infections in human populations in Africa. We further investigated if individuals infected with protozoan or helminth infection were more likely than uninfected individuals to present with a mental illness.
Materials and methods
Search methods
This study approach was a systematic review of published literature. A PRISMA (Page et al., Reference Page, McKenzie, Bossuyt, Boutron, Hoffmann, Mulrow, Shamseer, Tetzlaff, Akl, Brennsan, Chou and Moher2021) compliant systematic search of published data was performed in Embase, Global Health, Medline, PsycInfo, Pubmed and Web of Science (see Fig. 1). The search was conducted by four independent reviewers and includes studies published from 2000 to 2020. The search terms were as inclusive as possible and included those indicated in Fig. 2.

Fig. 1. Search terms used to find papers on parasite infection and mental illness.
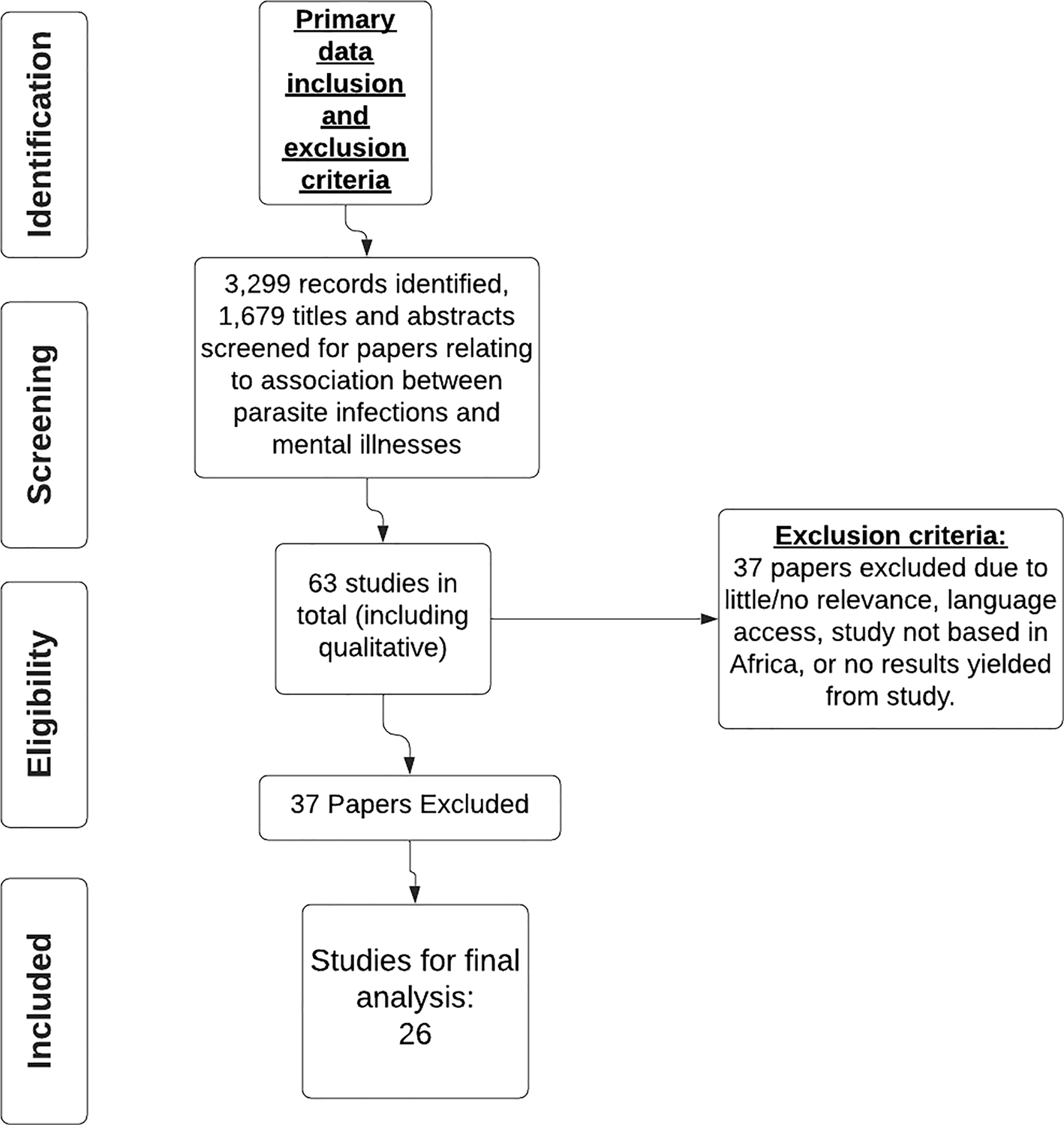
Fig. 2. PRISMA chart for inclusion and exclusion criteria on studies on parasite infection and mental illness.
Selection criteria
Abstracts were screened for selection if they included data on both parasite infections and mental illnesses in human populations. Data extracted included participants of any ethnicity, sex and age; educational attainment or sociodemographic factors were not controlled for as not all publications gave this information. The studies included are all based in African countries. Following the primary exclusion criteria 63 selected papers were saved to a bibliography in EndNote and were then reviewed using the secondary inclusion and exclusion criteria (see Fig. 2), leaving 26 papers for final analysis. Only papers published in English were considered. There were eight study types included in this review: cross-sectional, case controls, case series, case studies, systematic reviews, qualitative, observational/exploratory and pilot studies. This information was included in the data extracted.
The International Classification of Diseases 10 of Mental and Behavioural Disorders for researchers (ICD-10) (WHO, 1992) was used to classify the mental illnesses included in this review. Mood affective disorders (MAD) (ICD-10 codes F30-39) including all types of depression (mild, moderate, severe, recurrent and major depressive disorder) and bipolar disorder were included. Neurotic, stress-related disorders (NSRD) (ICD-10 codes F40-48), including anxiety (mild, moderate, severe, general and social), obsessive−compulsive disorder and post-traumatic stress disorder were included. Schizotypal disorders (s.d.) (ICD-10 codes F20-29) including schizophrenia, delusions, hallucinations and psychosis were also included. Unspecified mental illnesses (UMI), including stigmatization, low self-esteem, low quality of life (QoL) and health related QoL (HRQoL) were included, as most papers list such illnesses as afflicting the sample populations. All other types of mental illness were excluded. As the focus of this review was on mental illness, neurological disorders were excluded. Suicidal ideation was included under MAD, but suicide mortality was excluded.
The following protozoa parasite infections were considered: leishmaniasis caused by Leishmania donovani, L. infantum, or L. major, human African trypanosomiasis caused by Trypanosoma brucei gambiense and T. brucei rhodesiense, malaria caused by Plasmodium falciparum, P. malariae, P. vivax, P. ovale or P. knowlesi, toxoplasmosis caused by Toxoplasma gondii, and Chagas disease caused by Trypanosoma cruzi.
The following helminth parasite infection types were considered: helminthiasis caused by Ascaris lumbricoides, Trichuris trichiura, Ancylostoma duodenale or Necator americanus, schistosomiasis caused by Schistosoma mansoni or S. haematobium, lymphatic filariasis caused by Wuchereria bancrofti, Brugia malayi and B. timori, toxocariasis caused by Toxocara, nodding syndrome caused Onchocerca volvulus, dracontiasis caused by Dracunculus medinensis, onchocerciasis caused by Onchocerca volvulus and cysticercosis caused by Taenia solium.
Data extraction
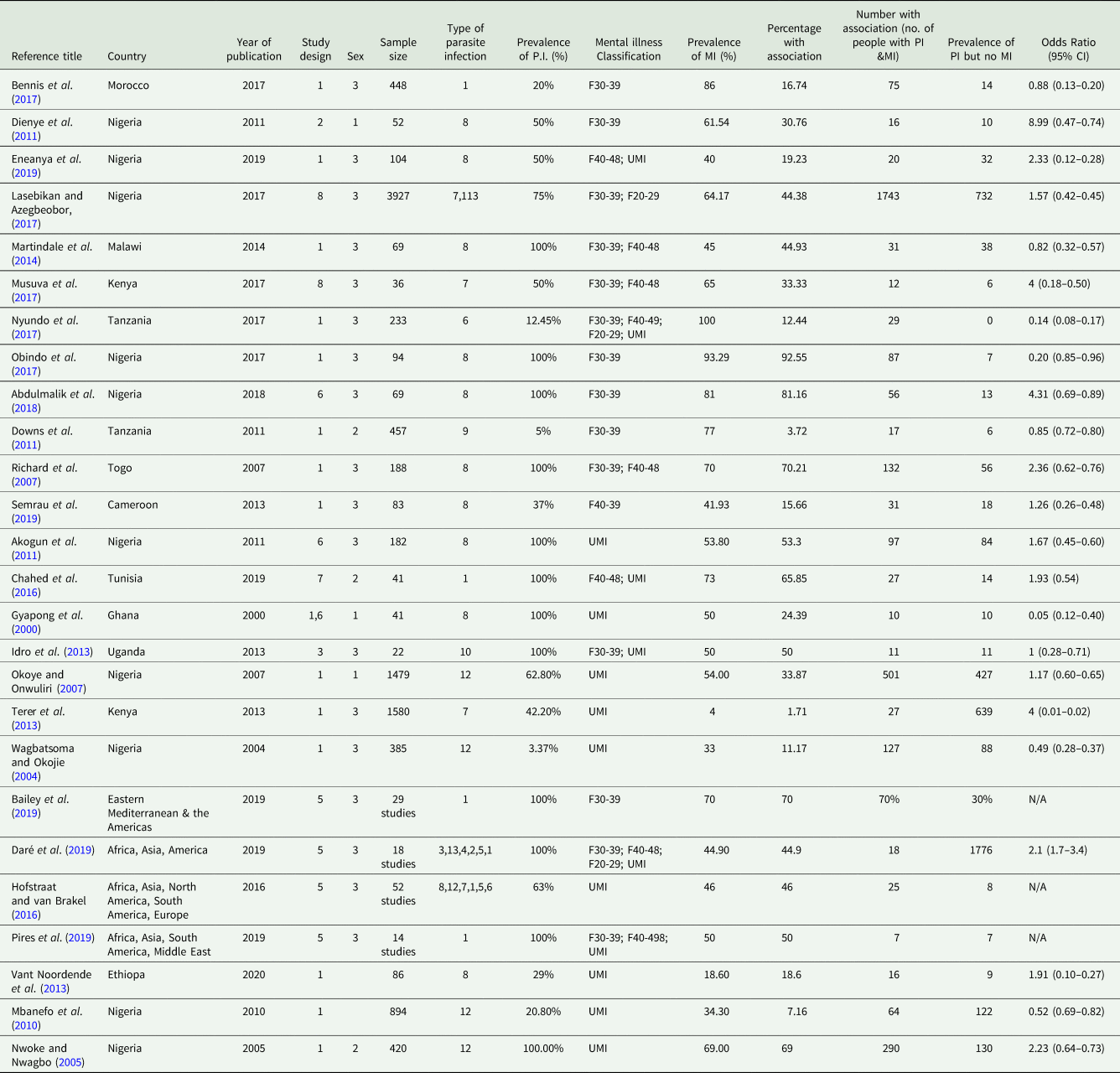
Data was extracted from all papers meeting the inclusion criteria and inputted into an Excel MS table. This process was repeated by the independent reviewers and the final datasheets compared and merged before analysis (see Table 5). The columns were organized as follows: Reference title; Reference number; Country and continent; Year of publication; Study design; Sex; Sample size; Type of parasitic infection; Percentage of participants with parasite infection; ICD-10 mental illness classification code; Percentage of participants with mental illness; Percentage of participants that have both a mental illness and parasitic infection; Number of participants with a parasitic infection and mental illness; Number of participants with a parasite infection but no mental illness; Odds ratio.
The type of parasite infection and mental illness of each study was inputted into the data extraction table. The number and percentage of association of those with both a parasite infection and mental illness were calculated for each study, giving one output detailing the overall prevalence of mental illness and parasite infection in each reviewed study.
Grading of studies (GRADE)
Papers presenting incidence or prevalence of any parasite infection and associated mental illness were evaluated using an adapted GRADE score (Guyatt et al., Reference Guyatt, Oxman, Vist, Kunz, Falck-Ytter, Alonso-Coello and Schünemann2008) (see Table 1) based on the following features: the type of diagnostic tool of parasite infection used, the type of diagnostic tool of mental illness used, sample size, year of study, type of publication (i.e. research paper, review paper or case study), methodology and the presence/absence of control group. Laboratory, clinical and/or imaging diagnostic tools score the highest as they are the gold standard and most reliable tool with the greatest assurance of validity in the results. Furthermore, studies that were published in the last 5 years score higher than those which were published in the last twenty-plus years, as results can differ with time, through changes to cultural/societal any of attitudes and diagnostic advances. Papers/ studies with a GRADE score of >7 out of a total of 16 were deemed to meet the minimal quality of information presented and were included in the analysis. Papers with a score of <7 were excluded. See Table 2 for the Modified GRADE score table for all studies included in this review.
Table 1. Scoring system for modified GRADE criteria

Table 2. Modified GRADE score for the papers in SR

Data synthesis
A descriptive analysis on the extracted data was performed on IBM SPSS v.24 and GraphPad Prism v.8.0. Descriptive statistics summarized the prevalence of parasite infection and any type of mental illness. Chi-square and Fisher's exact tests were utilized to determine if the frequency of mental illness differed significantly between people co-infected with a parasite and those free of parasitic infection across all papers. Odds ratios for each paper were calculated to ascertain the risk of developing a mental illness when testing positive for a parasite infection.
Non-parametric tests were utilized in the analysis of the data. The Kruskal–Wallis and the Mann–Whitney-U tests were performed to compare the differences in the prevalence of association for mental illness and parasite infection. This was conducted both for all parasites and all mental illnesses combined and then broken down by parasite infection type (protozoan and helminth) and by mental illness.
For papers not suitable for inclusion in statistical data analysis, Textual Narrative Synthesis (TNS) (Barnett-Page and Thomas, Reference Barnett-Page and Thomas2009) was utilized. TNS results were presented as a table summarizing the study characteristics, study design, methods, the diagnostic tools utilized to assess the presence of both parasite infection and mental illness, the prevalence of parasite infection and mental illness, the prevalence with an association of parasite infection and mental illness, the prevalence of association of parasite infection and no mental illness and the overall perceptions, understanding and expectations of mental illnesses associated with parasite infections and the main findings of the papers. These findings are reported to identify commonalities, themes and differences reported across the papers (Table 4).
Results
Systematic review
A total of 139 full-text papers were selected for a full review of which 63 passed the inclusion and exclusion criteria. Following the secondary inclusion and exclusion process, the 37 excluded papers were omitted due to little or no relevance to the aims of the review, no access to the papers due to language barriers, no results had been yielded from the paper as of yet, because the study was not based in Africa or due to a low-GRADE score (Wise et al., Reference Wise, Easom, Watson, Bailey and Brown2012; Goldner-Vukov et al., Reference Goldner-Vukov, Moore, Bayley, Abeysundera and Arunachalam2014), leaving 26 papers for final analysis. Figure 2 depicts the review process. The papers that met the inclusion criteria are detailed in Table 4. A full reference list is given in the bibliography.
The total population of persons included in all papers examined in this review was 14 856. The types of the study included were cross-sectional (15), case–control (1), case-series (1), a systematic review (4), qualitative (2), observational/exploratory (1) and pilot studies (2) (see Fig. 3a). The sample sizes ranged from 22 (Idro et al., Reference Idro, Opoka, Aanyu, Kakooza-Mwesige, Piloya-Were, Namusoke and Tumwine2013) to 3927 individuals (Lasebikan and Azegbeobor, Reference Lasebikan and Azegbeobor2017) (see Fig. 3c). The mean age was 29.55 (s.d. ± 16.19). The majority of papers investigated populations with both sexes; but three papers looked at male-only populations (Gyapong et al., Reference Gyapong, Gyapong, Weiss and Tanner2000; Okoye and Onwuliri, Reference Okoye and Onwuliri2007; Dienye et al., Reference Dienye, Gbeneol and Akani2011) and three papers looked at female-only populations (Nwoke and Nwagbo, Reference Nwoke and Nwagbo2005; Downs et al., Reference Downs, Mguta, Kaatano, Mitchell, Bang, Simplice and Fitzgerald2011; Chahed et al., Reference Chahed, Bellali, Ben Jemaa and Bellaj2016).

Fig. 3. Descriptive statistics of included studies (A) Number of publications as per study design (B) Number of publications per year (C) Spread of sample sizes of the reviewed papers.
Comparison of mental illness prevalence
The χ 2 test showed that the prevalence of mental illness among parasite-infected individuals, 58.2%, was significantly higher than those without parasitic infection 41.8%, (χ 2 (1) = 684.1, P < 0.001 (one-tailed)). The odds of individuals with a parasite infection subsequently developing a mental illness were found to have a likelihood of 4.11 [95% CI (1.916–4.406)].
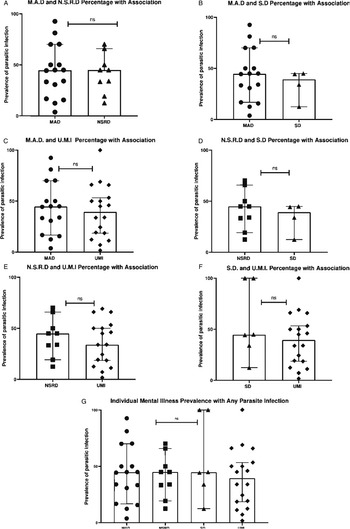
Looking at specific mental illness, neurotic stress-related disorder [Median = 44.90%, CI 95% (19.23–65.85), n = 9] was the most prevalent disorder among individuals carrying a parasite infection, followed jointly by mood affective disorder [Median = 44.64%, CI 95% (16.74–70.00), n = 16] and schizotypal disorder [Median = 44.64% (CI 95% 12.44–100), n = 6], then unspecified mental illness [Median = 39.45% (CI 95% 18.60–53.30), n = 18]. Nonetheless, there was no significant association between specific mental illnesses classifications and the presence of parasite infection [H (3) = 0.615, P = 0.96, see Fig. 4].

Fig. 4. Comparison of association between mental illness prevalence and parasite infection. (A) Mood affective disorders vs neurotic stress-related disorders (B) Mood affective disorders vs schizotypal disorders (C) Mood affective disorders vs unspecified mental illnesses (D) Neurotic stress-related disorders vs unspecified mental illnesses (E) schizotypal disorders vs unspecified mental illnesses (G) Specific mental illness prevalence of association with any parasite infection. Error bars represent 95% Confidence Intervals. Each data point represents the prevalence of parasitic infection in that mental illness group. Significance levels as displayed on graphs are abbreviated; abbreviations are as follows: ‘ns’ for not significant; for P levels (*P < 0.05).
From the papers that could not be analysed through statistical data analyses, using the TNS, mental illness was found to be highly associated with parasite infection in those afflicted. TNS observed high levels of stigmatization across the synthesized papers, which worked to aggravate the pre-existing mental illnesses and isolate persons afflicted with parasite infections. The key themes and commonalities relating to the stigmatization of parasite infection across the analysed papers were found to be fear of contagion, physical appearance, becoming a burden to family, becoming ostracized or shunned, loss of employment and inability to fulfil particular gender roles due to illness. A clear overlap in consequences related to parasite infections was also seen, in terms of social, economic, health-related and emotional concerns. Furthermore, lower QoL and HRQoL related to socioeconomic and emotional consequences of parasite infection, was often reported across the synthesized papers, as not only perpetuating the development of mental illness in the afflicted, but also increasing the global health burden of parasite infections overall (Table 4).
Comparison of mental illness prevalence of specific parasite infections with specific mental illnesses
There was no significant association observed from the Kruskal–Wallis test between specific parasite infection classes and the presence of any mental illness [H (3) = 3.42, P = 0.33, see Fig. 5a]. Nor was there a significant result yielded from the Kruskal–Wallis test examining specific parasitic infection type and specific mental illness classification [H (7) = 7.62, P = 0.367, see Fig. 5e]. There was insufficient data to compare the protozoa and schizotypal disorder (n = 1) and helminth and schizotypal disorder (n = 4). Conversely, there were no significant differences in the association between specific parasite infections and specific mental illnesses. However, there was a greater prevalence of UMI in individuals with protozoal infections compared to helminth infections (U = 12, P < 0.05, see Fig. 5b), although the small sample size of the protozoa group must be noted.

Fig. 5. Comparison of association between specific parasite infection and mental illness. (A) Comparison of association specific parasite infection classification and any mental illness. (B) Protozoa and unspecified mental illness vs Helminth and unspecified mental illness. (C) Protozoa and mood affective disorders vs Helminth and mood affective disorders. (D) Protozoa and neurotic stress-related disorder vs Helminth and neurotic stress-related disorder. (E) Comparison of association between specific parasite infection class and specific mental illness classifications. Error bars represent 95% Confidence Intervals. Each data point represents the prevalence of the specific parasitic infection and mental illness. Significance levels as displayed on graphs are abbreviated; abbreviations are as follows: ‘ns’ for not significant; for P levels (*P < 0.05).
Discussion
The primary purpose of this meta-analysis was to determine if the prevalence of mental illness was higher in people presenting with a parasite infection. From a systematic literature search, the 26 included studies revealed evidence of associations between parasitic infections and mental illness. Among people with parasitic infection, 58.2% had a mental illness compared to 41.8% of uninfected people with mental illnesses and the risk of an individual developing a mental illness was found to increase 4-fold when presenting with a parasitic infection. Helminth parasite infection types were separated from protozoan infection types as their mode of infection, physiology, niches and pathophysiology are different. These differences can potentially lead to different mechanistic pathways affecting the host's mental health. While protozoans are neurotropic, this is not a common phenomenon in helminths (with the exception of tapeworms causing cysticercosis). There are also secondary effects resulting from these biological differences between protozoa and helminths. For example, it has been previously shown that schistosome infections impact on the host's gut microbiome (Osakunor et al., Reference Osakunor, Munk, Mduluza, Petersen, Brinch, Ivens, Chimponda, Amanfo, Murray, Woolhouse and Mutapi2020) and there have been studies relating the gut microbiome structure to mental health via the gut−brain axis (Clapp et al., Reference Clapp, Aurora, Herrera, Bhatia, Wilen and Wakefield2017; Järbrink-Sehgal and Andreasson, Reference Järbrink-Sehgal and Andreasson2020).
The present findings are consistent with research showing direct and indirect associations between parasitic infection and mental illness. For example, T. gondii has been shown to play a role in several psychiatric disorders such as schizophrenia, bipolar disorder and MAD (Webster et al., Reference Webster, Lamberton, Donnelly and Torrey2006; Arling et al., Reference Arling, Yolken, Lapidus, Langenberg, Dickerson, Zimmerman and Postolache2009; de Barros et al., Reference de Barros, Barbosa, Salem, Rocha, Kummer, Okusaga and Teixeira2017).
Mechanistic pathways include the involvement of the host's immune response to the parasitic infections, particularly inflammatory responses in the potential development of psychiatric illnesses (Miller and Cohen, Reference Miller and Cohen2001; Raison et al., Reference Raison, Capuron and Miller2006; Capuron, and Miller, Reference Capuron and Miller2004; Borsini et al., Reference Borsini, Zunszain, Thuret and Pariante2015; Maizels and McSorley, Reference Maizels and McSorley2016; Miller and Raison, Reference Miller and Raison2016). Peripheral inflammation can affect the CNS via three mechanisms (Miller and Raison, Reference Miller and Raison2016). Firstly, the humoral pathway where cytokines in the blood pass through permeable regions such as the circumventricular of the blood−brain barrier. Secondly, direct activation of the vagus nerve activates ascending catecholamine pathways in the brain, resulting in central cytokine signalling. A third pathway involving the production and trafficking of neurotoxic metabolites of the tryptophan cascade, activated in both the brain and the liver has been identified from post-mortem studies (Dantzer et al., Reference Dantzer, O'Connor, Freund, Johnson and Kelley2008; Torres-Platas et al., Reference Torres-Platas, Cruceanu, Chen, Turecki and Mechawar2014). Such alterations to the immune system caused by parasitic infection and the loss of key immune cells such as T lymphocytes have been documented as having a negative impact on emotional well-being and cognition (Pariante, Reference Pariante2016).
Host genetics plays an important role in susceptibility to both parasitic infection (Alcaïs et al., Reference Alcaïs, Abel and Casanova2009) and in the prediction and regulation of depression (Bull et al., Reference Bull, Huezo-Diaz, Binder, Cubells, Ranjith, Maddock, Miyazaki, Alexander, Hotopf and Cleare2009). Parasite infections are well-known to cause increased immune activation, in which certain gene variants are associated with an increased risk of psychopathology; with immune genes being observed to have greater effects on behavioural outcomes than the so-called ‘psychiatry genes’ (Bufalino et al., Reference Bufalino, Hepgul, Aguglia and Pariante2013). This makes it difficult to determine the specific links between parasitic infection and mental illness for therapeutic intervention. As such, there is a need for more mechanistic studies in wider populations. It has been hypothesized and to an extent tested, that by treating disorders of the immune system, it is also possible to treat comorbid psychiatric disorders (Capuron and Miller, Reference Capuron and Miller2004; Harrison et al., Reference Harrison, Brydon, Walker, Gray, Steptoe, Dolan and Critchley2009; Pariante, Reference Pariante2016; Baumeister et al., Reference Baumeister, Ciufolini and Mondelli2016b). Adding anti-inflammatory medications alongside anti-depressants or anti-psychotics in patients with a psychiatric disorder, increases the efficacy of these medications (Fond et al., Reference Fond, Hamdani, Kapczinski, Boukouaci, Drancourt, Dargel, Oliveira, Le Guen, Marlinge, Tamouza and Leboyer2014; Na et al., Reference Na, Lee, Lee, Cho and Jung2014).
Regardless of the paucity of mechanistic studies, our review did highlight the public health and individual health impact of the co-morbidity between parasitic infection and mental illness. The social and health consequences of stigma related to parasite infections were strongly evidenced throughout the reviewed papers as contributing factors to the development of psychological disorders, primarily anxiety and depression. The studies reviewed and analysed in this meta-analysis indicated various factors that affect and predispose to both parasitic infections and mental health disorders. While increased immune activation and risk of developing a psychiatric disorder can be caused by genetic predispositions; environmental influences such as exposure to trauma, social inequities, stigmatization, urbanicity, deprivation and poor nutrition also play a key role in the development of psychopathologies, all of which tend to be highly prevalent in parasite endemic nations (Pariante, Reference Pariante2016). Exposure to trauma early in life is one of the most prolific causes of developing a psychiatric disorder, and it has been evidenced that early life trauma activates the immune system in young adults, even in the absence of psychopathology (Danese et al., Reference Danese, Pariante, Caspi, Taylor and Poulton2007). Individuals with increased immune activation and exposure to personal maltreatment, socioeconomic disadvantage, isolation and other forms of chronic stress have an increased likelihood of developing a psychiatric disorder in the future (Miller et al., Reference Miller, Chen, Sze, Marin, Arevalo, Doll, Ma and Cole2008; Danese et al., Reference Danese, Moffitt, Harrington, Milne, Polanczyk, Pariante, Poulton and Caspi2009; Miller et al., Reference Miller, Chen, Fok, Walker, Lim, Nicholls, Cole and Kobor2009; Baumeister et al., Reference Baumeister, Akhtar, Ciufolini, Pariante and Mondelli2016a, Reference Baumeister, Ciufolini and Mondelli2016b). Results from this review and that of empirical work suggest that there is a causational relationship between genetics/social factors and mental illnesses through immune-related mechanisms; that the relationship between inflammation caused by infection and mental illnesses is a blend of biological and environmental factors.
Across all studies included in this review, the stigma and general lack of understanding and acceptance of parasite infections was highly prevalent and reported across nearly all of the papers reviewed. The stigmatization of afflicted persons was seen to be a direct contributor of the development of psychological disorders, namely anxiety and depression (Hofstraat and van Brakel, Reference Hofstraat and van Brakel2016). Perhaps unsurprisingly, studies looking at parasite infections with highly visible characteristics, such as leishmaniosis scarring, leprosy, lymphatic filariasis and onchocerciasis, reported extremely high levels of stigma among their populations, with the reported effects of stigma being severe to the sufferer's mental wellbeing, with fears of social and familial rejection and socioeconomic strain exacerbating the toll of mental illness (Mbanefo et al., Reference Mbanefo, Eneanya, Nwaorgu, Oguoma, Otiji and Ogolo2010; Martindale et al., Reference Martindale, Mkwanda, Smith, Molyneux, Stanton and Kelly-Hope2014; Bennis et al., Reference Bennis, Thys, Filali, De Brouwere, Sahibi and Boelaert2017; Obindo et al., Reference Obindo, Abdulmalik, Nwefoh, Agbir, Nwoga, Armiya'u and Eaton2017; Bailey et al., Reference Bailey, Mondragon-Shem, Haines, Olabi, Alorfi, Ruiz-Postigo and Molyneux2019; Eneanya et al., Reference Eneanya, Garske and Donnelly2019; Pires et al., Reference Pires, Wright, Kaye, da Conceição and Churchill2019; van't Noordende et al., Reference van't Noordende, Aycheh and Schippers2020).
There were also some gender differences in the reporting of stigma across the reviewed papers. The majority of the studies included both genders, but those that examined men or women only yielded interesting results with regards to the differences and similarities faced by either gender when afflicted with a parasitic infection. Both men and women affected by parasite infection, were found to be less likely to marry or had higher rates of divorce. Mental illness, particularly depression, was found to be particularly prevalent across both men and women, often reported as a result of the severe stigmatization afflicted men and women experienced due to the physical manifestations of the parasite infection (e.g., hydrocele in men, and leishmaniasis in women). However, differences were also observed between men and women, with women reporting to experience higher levels of rejection and avoidance of stress and were less likely than men to promptly seek treatment (Chahed et al., Reference Chahed, Bellali, Ben Jemaa and Bellaj2016; Al-Kamel, Reference Al-Kamel2017).
The papers focusing on male-only populations were focused on the psychological and social effects of hydrocele (Gyapong et al., Reference Gyapong, Gyapong, Weiss and Tanner2000; Okoye and Onwuliri, Reference Okoye and Onwuliri2007; Dienye et al., Reference Dienye, Gbeneol and Akani2011) which revealed similar results, in that stigmatization of men with certain parasite infections was often debilitating and resulted in the development of depression. Furthermore, such men were less likely to wed and those already with a spouse were often divorced shortly thereafter. Overall, rates of depression were much more prevalent in men with conditions such as hydrocele and onchocerciasis compared to non-afflicted persons, with shame, low self-esteem, embarrassment and isolation as a result of their condition, being frequently reported. Such fears combined with perceived and actuated consequences of parasitic infection, are not only stigmatizing but are also conducive to developing a mental illness.
Similarly, studies examining the psychological and social effects of parasite infections on women only, reported similar effects (Downs et al., Reference Downs, Mguta, Kaatano, Mitchell, Bang, Simplice and Fitzgerald2011; Chahed et al., Reference Chahed, Bellali, Ben Jemaa and Bellaj2016). The scarring often caused by leishmaniasis has a particularly derogatory effect on young girls and women, who similarly to men, if they are unwed are much less likely to ever marry due to stigma (Yanik et al., Reference Yanik, Gurel, Simsek and Kati2004; Pires et al., Reference Pires, Wright, Kaye, da Conceição and Churchill2019), rejection and shunning from their community. The stigma experienced due to the dermatological aspects of leishmaniasis can hinder health-seeking behaviours in the afflicted, further exacerbating public health consequences (Al-Kamel, Reference Al-Kamel2017). Rates of depression tended to be very high in women afflicted by both leishmaniasis, as well as female urogenital schistosomiasis, with depression and any other psychological disorders experienced, often being accredited to the stigma anticipated and experienced by such women (Grifferty et al., Reference Grifferty, Shirley, McGloin, Kahn, Orriols and Wamai2021). However, the psychological effects of leishmaniasis were reported to reduce as women got older, with the greatest psychosocial burden seen to be on younger women reporting to experience stigma more frequently than older women (Chahed et al., Reference Chahed, Bellali, Ben Jemaa and Bellaj2016; Bennis et al., Reference Bennis, Thys, Filali, De Brouwere, Sahibi and Boelaert2017). Furthermore, the more educated an individual is on their disease, the greater their cognitive (perception of consequences) and emotional (emotional representation) sensitivity is (Pires et al., Reference Pires, Wright, Kaye, da Conceição and Churchill2019), thus reducing the effects of stigma on depression and anxiety for afflicted women due to improved education and understanding.
Limitations
The studies included in the analysis were heterogeneous in terms of study design, parasite types and mental illness and the sample size meant these factors and potential confounders could not be allowed for in the analyses. Similarly small sample sizes for some of the mental illnesses and parasites reduced the statistical power to detect any significant differences. As many studies as possible were included through TNS.
Conclusions/future directions
The results from this review suggest that there is a significantly increased risk of developing a mental illness when testing positive for a parasitic infection, however little evidence was found to imply that specific parasitic infections have a greater likelihood of leading to the development of specific mental illnesses. Taking together the results of this review and that of empirical work, it can be inferred that there is a potentially causational relationship between parasite infection and mental illness, through a blend of biological and environmental factors borne of an interplay between genetic, immunological, environmental and social influences. Whilst inflammation poses as a potential contributor and offers a prospective explanation for the development of mental illness, mental illnesses are complex conditions which ultimately do not have one single cause, aetiology or treatment. Questions remain to be answered in future investigations, and further research is required to ascertain the exact nature of the association between parasitic infection and mental illness with greater clarification on the proportion of mental illnesses that can be attributed to inflammation caused by parasite infection. The study highlights the public health importance of addressing both health challenges simultaneously for improved human health.
Data
The GRADE classification system and the PRISMA checklist and flow diagrams were used to improve the reporting of this systematic review to ensure validity in data extraction and analyses. The International Classifications of Diseases 10 of Mental and Behavioural Disorders for researchers was used to classify the mental disorders included in this review.
See Table 1 for the GRADE scoring system, Table 2 for the reviewed papers GRADE scores, Table 3 for the data table used for the Chi-Square test and PAF, Table 4 for the TNS results and Table 5 for the final data extraction table. See Fig. 6 for the PRISMA chart and for the studies excluded from the final analysis based on secondary exclusion criteria.

Fig. 6. Studies excluded from the final analysis based on secondary exclusion criteria.
Table 3. Data table for chi-square and Fisher's exact test

Table 4. Textual narrative synthesis table

Table 5. Final Data Extraction Table by ALS
Acknowledgements
We thank the Parasite Immunoepidemiology Group at the University of Edinburgh and their feedback on early drafts of the manuscript.
Author contribution
ALS- conducted the literature review, data extraction, data analysis and prepared the first draft of the manuscript. JK, RI, AM all conducted independent literature reviews, data extraction and preliminary data analyses. FM conceived of the idea, supervised the work and co-wrote the first draft of the manuscript. All co-authors read and approved the final manuscript before submission.
Financial support
This research was commissioned by the National Institute for Health Research (NIHR) Global Health Research programme (16/136/33) using UK aid from the UK Government. The views expressed in this publication are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
Conflicts of interest
The authors declare no conflict of interest.