Article contents
Fibronectin degradation as biomarker for Trypanosoma cruzi infection and treatment monitoring in mice
Published online by Cambridge University Press: 24 May 2021
Abstract
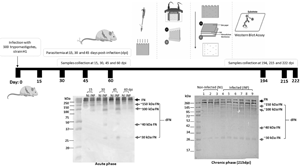
Biomarkers (coming from host or parasite) to monitor Chagas disease (CD) progression as well as the therapeutic response in chronic CD are critically needed, since seronegativization, which may be considered the best indicator of therapeutic cure, takes several years to be observed in adults. Several molecules have been suggested as biomarkers for CD, however, they have to be validated. Taking advantage of mouse models of Trypanosoma cruzi infection, we investigated changes in the degradation profile of fibronectin in plasma. The degradation profile of fibronectin was different in the acute phase compared to the chronic phase of the infection. Fibronectin fragments of approximately 150, 100, 40 and 30 kDa were identified. Furthermore, those degradation profiles correlated with acute parasitaemia as well as with cardiac parasite burden and tissue damage during the infection. The usefulness of fibronectin degradation as a biomarker for therapeutic response following drug treatment and immunotherapeutic vaccination also was evaluated and a decreased fibronectin degradation profile was observed upon benznidazole or a vaccine candidate treatment.
- Type
- Research Article
- Information
- Copyright
- Copyright © The Author(s), 2021. Published by Cambridge University Press
References
- 3
- Cited by



