Introduction
Toxoplasma gondii, Neospora caninum and Besnoitia besnoiti are cyst-forming species belonging to the Apicomplexa phylum, which consists of a large group of obligatory intracellular protozoan parasites that affect both humans and animals. Despite morphological similarities between coccidian species, host specificity and clinical consequences greatly differ among them. In this context, T. gondii is considered a major public health problem and an abortive agent especially in ovines (Benavides et al., Reference Benavides, Fernandez, Castano, Ferreras, Ortega-Mora and Perez2017) and humans (Nayeri et al., Reference Nayeri, Sarvi, Moosazadeh, Amouei, Hosseininejad and Daryani2020). The closely related coccidian parasite N. caninum is currently considered as a major cause of abortions in cattle (Reichel et al., Reference Reichel, Alejandra Ayanegui-Alcérreca, Gondim and Ellis2013). In contrast, B. besnoiti causes bovine besnoitiosis, an emerging disease within Europe, which is characterized by massive alterations of skin and mucosas and also bull infertility (Alvarez-Garcia et al., Reference Alvarez-Garcia, Frey, Mora and Schares2013).
During the acute stage of infection, coccidian parasites undergo asexual replication within host cells. In this context, host endothelial cells have shown high permissiveness for tachyzoite infection and proliferation in vivo (Alvarez-Garcia et al., Reference Alvarez-Garcia, Frey, Mora and Schares2013; Konradt et al., Reference Konradt, Ueno, Christian, Delong, Pritchard, Herz, Bzik, Koshy, McGavern, Lodoen and Hunter2016). Likewise, primary bovine endothelial cells have consistently been reported as suitable for in vitro replication of T. gondii, N. caninum and B. besnoiti (Taubert et al., Reference Taubert, Zahner and Hermosilla2006, Reference Taubert, Hermosilla, Silva, Wieck, Failing and Mazurek2016; Silva et al., Reference Silva, Lutjohann, Hamid, Velasquez, Kerner, Larrazabal, Failing, Hermosilla and Taubert2019; Velásquez et al., Reference Velásquez, Conejeros, Larrazabal, Kerner, Hermosilla and Taubert2019), allowing high tachyzoite proliferation rates in an experimental set up close to the in vivo scenario. During the fast proliferation phase, tachyzoites need significant amounts of nutrients for offspring development, which may be obtained from the host cell or newly synthesized. Specifically during coccidian replication high amounts of cholesterol are needed for new membrane biosynthesis (Coppens, Reference Coppens2013). Given that apicomplexan parasites are considered auxotrophic for cholesterol (Coppens, Reference Coppens2013), their replication within the parasitophorous vacuole (PV) highly depends on cholesterol supply by the host cell. In general, cellular cholesterol supply may be achieved either by enhancement of cellular endogenous de novo biosynthesis or by an increased cholesterol uptake from extracellular sources (Luo et al., Reference Luo, Yang and Song2020). In line, apicomplexan parasites can differentially exploit cholesterol sources depending on host cell type and parasite species. LDL internalization appears the main pathway for cholesterol uptake, and cholesterol esterification allows for storage in lipid-rich organelles (Luo et al., Reference Luo, Yang and Song2020). Recently, LDL-mediated cholesterol incorporation was described as pivotal, but not exclusive mechanism to fulfil cholesterol requirement during fast replicating coccidia proliferation (Nolan et al., Reference Nolan, Romano, Luechtefeld and Coppens2015; Silva et al., Reference Silva, Lutjohann, Hamid, Velasquez, Kerner, Larrazabal, Failing, Hermosilla and Taubert2019).
Based on pathophysiological consequences of human hyperlipidaemia, several pharmacological lipid-lowering compounds have been developed (Barter and Rye, Reference Barter and Rye2016). Amongst these, ezetimibe is one of the most common hypolipidaemic drugs, which is capable of reducing intestinal cholesterol absorption by its interaction with Niemann−Pick C-1 like-1 protein (NPC1L1) in enterocytes (Davis et al., Reference Davis, Zhu, Hoos, Tetzloff, Maguire, Liu, Yao, Iyer, Lam, Lund, Detmers, Graziano and Altmann2004; Garcia-Calvo et al., Reference Garcia-Calvo, Lisnock, Bull, Hawes, Burnett, Braun, Crona, Davis, Dean, Detmers, Graziano, Hughes, Macintyre, Ogawa, O'Neill K, Iyer, Shevell, Smith, Tang, Makarewicz, Ujjainwalla, Altmann, Chapman and Thornberry2005). In detail, ezetimibe binds to NPC1L1, resulting in the blockage of NPC1L1 endocytosis into clathrin-coated vesicles and thereby diminishing cholesterol internalization into enterocytes (Ge et al., Reference Ge, Wang, Qi, Miao, Cao, Qu, Li and Song2008; Wang et al., Reference Wang, Chu, Ge, Li, Yan and Song2009). Despite that, the participation of other potential ezetimibe targets as the class B type 1 scavenger receptor (SR-BI) and the aminopeptidase N (CD13) have been linked to its hypolipidaemic effect (Kramer et al., Reference Kramer, Girbig, Corsiero, Pfenninger, Frick, Jähne, Rhein, Wendler, Lottspeich, Hochleitner, Orsó and Schmitz2005; Labonté et al., Reference Labonté, Howles, Granholm, Rojas, Davies, Ioannou and Hui2007). In general, the efficacy and safety of ezetimibe has been reported in mice and human studies (Bays et al., Reference Bays, Moore, Drehobl, Rosenblatt, Toth, Dujovne, Knopp, Lipka, Lebeaut, Yang, Mellars, Cuffie-Jackson, Veltri and Ezetimibe Study2001; van Heek et al., Reference van Heek, Farley, Compton, Hoos and Davis2001). As such, ezetimibe might represent a promising anti-parasitic drug candidate (Andrade-Neto et al., Reference Andrade-Neto, Cunha-Junior, Canto-Cavalheiro, Atella, Fernandes, Costa and Torres-Santos2016). In line, ezetimibe treatments significantly reduced Cryptosporidium parvum growth in Caco-2 cells (Ehrenman et al., Reference Ehrenman, Wanyiri, Bhat, Ward and Coppens2013). However, other evidences are incongruent: whilst ezetimibe reduced the parasite burden of Leishmania amazonensis in vivo, and diminished the L. infantum replication in vitro and in vivo (alone or in binary and ternary combination with miltefosine and itraconazole) (Andrade-Neto et al., Reference Andrade-Neto, Cunha-Junior, Canto-Cavalheiro, Atella, Fernandes, Costa and Torres-Santos2016, Reference Andrade-Neto, Rebello, Pereira and Torres-Santos2021), this treatment did not affect Plasmodium yoelii parasitaemia in mice, while reduced the intraerythrocytic proliferation of P. falciparum in vitro (Kume et al., Reference Kume, Herbas, Shichiri, Ishida and Suzuki2016; Hayakawa et al., Reference Hayakawa, Kato, Nardone and Usukura2021), thereby suggesting parasite-specific effects for this compound.
So far, no data are available on the impact of this drug on typical fast replicating coccidian parasites. Therefore, the aim of this study was to evaluate anti-parasitic efficacy of ezetimibe in T. gondii, N. caninum and B. besnoiti-infected primary bovine host endothelial cells.
Materials and methods
Host cell culture
Primary bovine umbilical vein endothelial cells (BUVEC) were isolated as described elsewhere (Taubert et al., Reference Taubert, Zahner and Hermosilla2006). BUVEC were cultured at 37°C and 5% CO2 atmosphere in modified endothelial cell growth medium (modECGM), by diluting ECGM medium (Promocell®) with M199 (Sigma-Aldrich) at a ratio of 1:3, supplemented with 500 U/mL penicillin (Sigma-Aldrich) and 50 μg/mL streptomycin (Sigma-Aldrich) and 5% FCS (foetal calf serum; Biochrom). BUVEC of less than three passages were used in this study.
Parasites
Toxoplasma gondii (strain RH) and Neospora caninum (strain NC-1) tachyzoites were cultivated in vitro as described elsewhere (Taubert et al., Reference Taubert, Zahner and Hermosilla2006; Velásquez et al., Reference Velásquez, Conejeros, Larrazabal, Kerner, Hermosilla and Taubert2019), by maintaining them at several passages in permanent African green monkey kidney epithelial cells (MARC 145) in Dubelcco's modified eagle medium (DMEM) (Sigma-Aldrich). Besnoitia besnoiti (strain Bb Evora04) tachyzoite stages were propagated in Madin−Darby bovine kidney cells (MDBK) (Velásquez et al., Reference Velásquez, Lopez-Osorio, Pervizaj-Oruqaj, Herold, Hermosilla and Taubert2020) in Roswell Park Memorial Institute (RPMI) medium (Sigma-Aldrich). All culture media were supplemented with 500 U/mL penicillin and 50 μg/mL streptomycin and 5% foetal calf serum (FCS; Sigma-Aldrich). Infected and non-infected cells were cultured at 37°C and 5% CO2 atmosphere. Vital tachyzoites were collected from supernatants of infected host cells (800 × g; 5 min) and re-suspended in modECGM for further experiments.
For infection rate-related experiments, tachyzoites of each species were pre-incubated in 20 μ m ezetimibe for 1 h. After washing in modECGM (800 × g; 5 min), tachyzoites were used for infection experiments.
Treatments of host cells and infections
BUVEC (n = 5) were seeded in 12-well plates (Sarstedt) pre-coated with fibronectin (1:400; Sigma-Aldrich). Ezetimibe (Cayman Chemical) and ezetimibe-glucuronide (Santa Cruz Biotechnology) stock solutions were prepared in dimethyl sulphoxide (DMSO; Sigma-Aldrich, 33 mm), diluted in modECGM at 2.5, 5, 10 and 20 μ m and administered to fully confluent cell monolayers 48 h before infection. ModECGM with DMSO (0.06%) served as vehicle control. Following pre-treatments, the medium was entirely removed and cells were infected with tachyzoites of T. gondii, B. besnoiti or N. caninum at a multiplicity of infection of 1:5 for 4 h under inhibitor-free conditions. Then, extracellular tachyzoites were removed and fresh medium with inhibitors was re-administered. At 4 h post infection (p. i.), phase-contrast images for infection rate estimation [(infected cells/total cells) × 100] were acquired by an inverted microscope (IX81, Olympus®) equipped with a digital camera (XM10, Olympus®). Tachyzoites present in cell culture supernatants were collected (800 × g; 5 min) at 48 h p. i. and counted in a Neubauer chamber.
Additionally, withdrawal experiments were carried out. Therefore, ezetimibe-containing medium was replaced by control medium at 24 h p. i., and parasite replication was estimated 24 h later (n = 5). Finally, further assays were performed to estimate the effect of ezetimibe over time as cells were treated as described above with a daily replacement of medium containing ezetimibe (20 μ m) at 24, 48 and 72 h p. i., and tachyzoite proliferation was observed at 48, 72 and 96 h p. i., respectively (n = 5).
Live cell 3D holotomographic microscopy to illustrate parasite development
BUVEC were seeded into 35 mm tissue culture μ-dishes (Ibidi®) and cultured (37°C, 5% CO2) until confluence. Ezetimibe treatment (20 μ m) was performed as described above. Thereafter, T. gondii, N. caninum and B. besnoiti tachyzoites were used to infect cell layers (MOI = 3:1). At 24 h p. i., holotomographic images were obtained by using 3D Cell-Explorer-fluo microscope (Nanolive) equipped with a 60 × magnification (λ = 520 nm, sample exposure 0.2 mW/mm2) and a depth of field of 30 μ m. Images were analysed using STEVE software (Nanolive) to obtain refractive index (RI)-based z-stacks (Silva et al., Reference Silva, Lutjohann, Hamid, Velasquez, Kerner, Larrazabal, Failing, Hermosilla and Taubert2019). Additionally, digital staining was applied according to the RI of intracellular tachyzoites. Finally, intracellular meront development was evaluated by counting intra-meront tachyzoites in at least six 3D holotomographic z-stacks of infected host cells ( = 50 cells per condition) in presence or absence of ezetimibe (20 μ m).
RT-qPCR for relative quantification of NPC1L1 mRNA
BUVEC (n = 5) grown in 25 cm2 culture tissue flasks (Greiner Bio-One) were infected with T. gondii, N. caninum or B. besnoiti tachyzoites (MOI = 5:1). Infected- and non-infected host cells were processed for total RNA isolation at four different time points after infection (3, 6, 12, 24 h p. i.). Tissue samples from bovine small intestine obtained at a local slaughterhouse were used as positive controls for NPC1L1. For total RNA isolation, the RNeasy kit (Qiagen) was used according to the manufacturer's instructions. Total RNAs were stored at −80°C until further use. In order to remove any genomic DNA leftover, DNA digestion step was performed. Therefore, 1 μg of total RNA was treated with 10 U DNase I (Thermo Scientific) in 1× DNase reaction buffer (37°C, 30 min). DNase was inactivated by heating the samples (65°C, 10 min). The efficiency of genomic DNA digestion was confirmed by no-RT-controls in each RT-qPCR experiment. cDNA synthesis was performed using the SuperScript IV (InvitrogenTM) according to the manufacturer's instructions. Briefly, for first-strand cDNA synthesis, 1 μg of DNase treated total RNA was added to 0.5 μL of 50 μ m oligo(dt), 1 μL of 50 ng/μL random hexamer primer, 1 μL of 10 mm dNTP mix in a total volume of 10 μL. Thereafter, the samples were incubated at 65°C for 5 min and then immediately cooled on ice. Additionally, 4 μL of 5× SSIV buffer, 1 μL 0.1 m DTT, 1 μL RNAse free H2O and 0.5 μL SuperScript IV enzyme were added obtaining a total volume of 20 μL. The samples were incubated at 23°C for 10 min followed by 50°C for 10 min and an 80°C inactivation step for 10 min.
Probes were labelled at the 5′-end with a reporter dye FAM (6-carboxyfluorescein) and at the 3′-end with the quencher dye TAMRA (6-carboxytetramethyl-rhodamine). bNPC1L1 primer sequences were designed as follows: Bos taurus NPC1L1 forward 5′- CTTCCCTGATATGTCTTAC −3′; reverse 5′- GACCAGAGATATAAAGGC-3′ probe AGCCAGTCAATGAAGTCGTCCA. qPCR amplification was performed on a Rotor-Gene Q Thermocycler (Qiagen) in duplicates in a 10 μL total volume containing 400 nm forward and reverse primers, 200 nm probe, 10 ng cDNA and 5 μL 2× PerfeCTa qPCR FastMix (Quanta Biosciences). The reaction conditions were as follows: 95°C for 10 min, 40 cycles at 95°C for 10 s, 60°C for 15 s and 72°C for 30 s. No-template controls and no-RT reactions were included in each experiment. As reference gene GAPDH was used as previously reported (Taubert et al., Reference Taubert, Zahner and Hermosilla2006; Hamid et al., Reference Hamid, Hirzmann, Hermosilla and Taubert2014; Hamid et al., Reference Hamid, Hirzmann, Kerner, Gimpl, Lochnit, Hermosilla and Taubert2015).
Viability assessment
For experiments on parasite viability, 5 × 105 tachyzoites of each parasite species were treated for 1 h with vehicle (DMSO 0.06%) or ezetimibe (20 μ m) (37°C, 5% CO2). Thereafter, viability of tachyzoites was determined by the trypan blue (Sigma-Aldrich®) exclusion staining assay as described elsewhere (Cervantes-Valencia et al., Reference Cervantes-Valencia, Hermosilla, Alcalá-Canto, Tapia, Taubert and Silva2019). Non-stained parasites were considered as viable. Additionally, cell viability after compound treatments was assessed by the colorimetric XTT test (Promega®) according to the manufacturer instructions. Briefly, BUVEC seeded in 96-well plate (Greiner) were incubated with DMSO, ezetimibe or ezetimibe-glucuronide (both 20 μ m) in a total volume of 50 μL for 72 h. Thereafter, 50 μL of XTT working solution was added, and samples were incubated for 4 h (37°C, 5% CO2 atmosphere). The resulting formazan product was estimated via optical density (OD) measurements at 590 nm and reference filter 620-nm wavelength using VarioskanTM Flash Multimode Reader (Thermo Scientific).
Statistical analysis
For statistical analyses, the statistical software GraphPad® Prism 8 (version 8.4.3.) was used. Data description was performed by presenting arithmetic mean ± standard deviation. In addition, the non-parametric statistical test Mann−Whitney for comparison of two experimental conditions was applied. In cases of three or more conditions, Kruskal−Wallis test was used. Whenever global comparison by Kruskal−Wallis test indicated significance, post hoc multiple comparison tests were carried out by Dunn tests to compare test with control conditions. Outcomes of statistical tests were considered to indicate significant differences when P ⩽ 0.05 (significance level).
Results
Ezetimibe treatments effectively block T. gondii, N. caninum and B. besnoiti tachyzoite proliferation
To analyse the effects of ezetimibe on intracellular tachyzoite replication, functional inhibition experiments were performed, thereby evaluating the number of freshly released tachyzoites at 48 h p. i. from cells pre-treated and exposed to ezetimibe during the intracellular parasite proliferation stage. Overall, ezetimibe treatments significantly inhibited tachyzoite replication of T. gondii (10 μ m, P = 0.0397; 20 μ m, P = 0.0010; Figure 1A), N. caninum (20 μ m, P = 0.0078; Figure 1B) and B. besnoiti (10 μ m, P = 0.0059; 20 μ m, P < 0.0001; Figure 1C) in BUVEC in a dose-dependent manner. Overall, the strongest effect of ezetimibe treatments at 20 μ m was observed in case of B. besnoiti (99.2 ± 0.5% replication reduction), followed by T. gondii (95.7 ± 2.3% reduction) and N. caninum (73.1 ± 2.8% reduction). In line, phase-contrast microscopy showed an impairment in meront development for T. gondii- (Fig. 1A1 and 1A2), N. caninum- (Fig. 1B1 and 1B2) and B. besnoiti- (Fig. 1C1 and 1C2) infected BUVEC at 24 h p. i. To better visualize ezetimibe-based effects on parasite development, additionally live cell 3D holotomographic microscopy were performed. As illustrated in Fig. 2, treatments with ezetimibe led to reduced meront sizes in T. gondii, N. caninum and B. besnoiti infections (Fig. 2), without apparently affecting the morphology of non-infected host cells (data not shown). Additionally, the number of tachyzoites per PV was determined to better understand ezetimibe-derived impact on parasite development (Fig. 2). Ezetimibe treatments markedly reduced the number of tachyzoites per meront in all three parasite species (all: P < 0.0001), however, the strongest effect was observed for B. besnoiti, with a reduction of 68.2% on the mean number of tachyzoites per meront, followed by T. gondii and N. caninum showing more than 50% reduction (56.5% and 50.2%, respectively).

Fig. 1. Ezetimibe treatments inhibit T. gondii, N. caninum and B. besnoiti tachyzoite proliferation in primary endothelial cells. BUVEC were treated with ezetimibe (2.5, 5, 10 and 20 μ m) 48 h before (A) T. gondii, (B) N. caninum or (C) B. besnoiti infection (MOI 1:5). 48 h after infection, the number of tachyzoites present in cell culture supernatants were counted (A–C). Exemplary illustration of T. gondii (A1−A2) N. caninum (B1−B2) or B. besnoiti (C1−C2) meront development at 24 h post infection. Scale bar represents 5 μ m. Bars represent means of five biological replicates ± standard deviation. * P ⩽ 0.05; ** P ⩽ 0.01; *** P ⩽ 0.001; **** P ⩽ 0.0001.

Fig. 2. Ezetimibe treatment affects intracellular meront formation and reduces the number of T. gondii, N. caninum and B. besnoiti intra-meront tachyzoites. Ezetimibe-pretreated BUVEC were infected with T. gondii, N. caninum and B. besnoiti tachyzoites and live cell 3D holotomographic microscopy was performed at 24 h p. i. Digital staining was achieved via STEVE software analysis. Violin plots depict the distribution of absolute T. gondii, N. caninum and B. besnoiti tachyzoite number per meront.
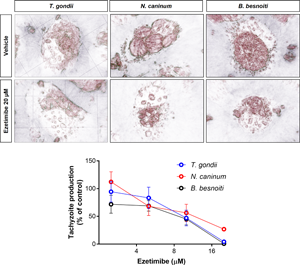
Fast replicating coccidian fulfil their replication cycle within 36–48 h p. i. in BUVEC layers in vitro (Taubert et al., Reference Taubert, Zahner and Hermosilla2006; Silva et al., Reference Silva, Lutjohann, Hamid, Velasquez, Kerner, Larrazabal, Failing, Hermosilla and Taubert2019; Velásquez et al., Reference Velásquez, Conejeros, Larrazabal, Kerner, Hermosilla and Taubert2019). In this context, the sustained inhibitory effect of ezetimibe over time was evaluated by counting tachyzoite production daily at 48, 72 and 92 h p. i. As depicted in Fig. 3, ezetimibe (20 μ m) effectively blocked T. gondii (99.1 ± 0.0% reduction; A1−A4), N. caninum (75.9 ± 7.6% reduction; B1−B4) and B. besnoiti (99.6 ± 0.1% reduction; C1–C4) replication over time (48, 72 and 96 h p. i.).

Fig. 3. Ezetimibe blocks T. gondii, N. caninum and B. besnoiti tachyzoite proliferation over time. Effect of daily ezetimibe treatments on tachyzoite proliferation over time: BUVEC were treated with ezetimibe (20 μ m) 48 h before infection and then infected with T. gondii (A), N. caninum (B) and B. besnoiti (C) tachyzoites. Exemplary live cell 3D holotomographic illustration of T. gondii (A1−A3) N. caninum (B1−B3) or B. besnoiti (C1−C3) meront development (arrows) at 48, 72 and 96 h p. i., respectively. At 48, 72 and 96 h p. i., the number of tachyzoites present in cell culture supernatants were counted (A4, B4, C4). Bars represent means of five biological replicates ± standard deviation. * P ⩽ 0.05; ** P ⩽ 0.01; *** P ⩽ 0.001; **** P ⩽ 0.0001.
To estimate whether ezetimibe induces either parasitostatic or parasitocidal effects, compound withdrawal experiments were performed at 24 h p. i. As illustrated in Fig. 4, remnant T. gondii and N. caninum tachyzoites quickly recovered and regained proliferative capacities 24 h after ezetimibe withdrawal. In contrast, B. besnoiti proved more sensitive for ezetimibe treatments showing an ongoing reduction (31.6 ± 5.3%) of tachyzoite production when compared to non-treated cells (P = 0.15).

Fig. 4. Ezetimibe withdrawal restores T. gondii and N. caninum tachyzoite replication but hardly affects B. besnoiti recovery. Ezetimibe-treated BUVEC were infected with T. gondii, N. caninum and B. besnoiti tachyzoites. At 24 h p. i., ezetimibe was removed from cultures and tachyzoites present in supernatants 24 h after withdrawal were counted. Bars represent means of five biological replicates, standard deviation. * P ⩽ 0.05; ** P ⩽ 0.01; *** P ⩽ 0.001; **** P ⩽ 0.0001.
Ezetimibe treatments reduce tachyzoite infectivity but fail to affect host cell permissiveness
To fulfil intracellular replication tachyzoites must first actively invade the host cells. To determine if anti-parasitic effects of ezetimibe also relied on reduced infection rates, both compartments, i. e. host cells and parasites, were separately treated with ezetimibe and then tested for infection rates 4 h after infection. Therefore, BUVEC were pre-treated with ezetimibe for 48 h before infection. At 4 h p. i. non-treated control cells presented an infection rate of 50.2% (Fig. 5A), 51.8% (Fig. 5B) and 47.0% (Fig. 5C), for T. gondii, N. caninum and B. besnoiti, respectively. In pre-treated cells, similar infection rates were observed for each parasite species (Fig. 5A–C), thereby denying any effect of ezetimibe pre-treatments. In contrast, ezetimibe pre-treatments of fresh tachyzoites significantly reduced invasive capacities of T. gondii (P = 0.0079; Figure 5A), N. caninum (P = 0.0159; Figure 5B) and B. besnoiti (P = 0.0079; Figure 5C) tachyzoites, when compared to non-treated control stages. Here, species-dependent effects were observed since the impact of ezetimibe pre-treatments were more prominent in case of B. besnoiti (28.1% reduction) than in T. gondii (22.2% reduction) or N. caninum (17.3% reduction).

Fig. 5. Ezetimibe treatment affects the infection capacity of T. gondii, N. caninum and B. besnoiti tachyzoites. Non-treated or ezetimibe-treated BUVEC were infected with ezetimibe-treated or non-treated T. gondii (A), N. caninum (B) and B. besnoiti (C) tachyzoites. After 4 h, infection rates were estimated. Bars represent infection rate mean of five biological replicates ± standard deviation. * P ⩽ 0.05; ** P ⩽ 0.01.
Ezetimibe glucuronidation causes loss of anti-parasitic efficacy
Ezetimibe-glucuronide is the major and pharmacologically active metabolite of ezetimibe following in vivo liver biotransformation. Thus, a functional assay to evaluate the effect of this chemically modified molecule on tachyzoite proliferation in vitro was performed (Fig. 6). Here, only ezetimibe but not its glucuronated derivative led to a reduction of T. gondii, N. caninum nor B. besnoiti tachyzoite proliferation.

Fig. 6. Ezetimibe-mediated anti-parasitic effects are abolished by glucoronation. Ezetimibe- or ezetimibe-glucoronide-pre-treated BUVEC were infected with T. gondii (A), N. caninum (B) and B. besnoiti (C) tachyzoites. 48 h after infection, the number of tachyzoites present in cell culture supernatants were counted. Bars represent means of five biological replicates ± standard deviation. * P ⩽ 0.05; ** P ⩽ 0.01; *** P ⩽ 0.001; **** P ⩽ 0.0001.
NPC1L1 gene is inconsistently transcribed in T. gondii-, N. caninum- and B. besnoiti-infected BUVEC
Given that NPC1L1 is described as the main target of ezetimibe in humans, the profile of gene transcription of NPC1L1 was estimated over infection kinetics (3–24 h p. i.) on T. gondii-, N. caninum- and B. besnoiti-infected BUVEC by qRT-PCR. Using bovine small intestine tissue samples, the functionality of the qPCR system was proved. However, infection-related data showed that neither T. gondii-, N. caninum- and B. besnoiti-infected BUVEC nor non-infected controls have a reliable amplification of NPC1L1 mRNAs. Specifically, as illustrated in supplementary Table 1, NPC1L1 was not detected in a consistent manner, thereby showing amplification only in some of the replicates at a rather high threshold cycle (CT > 30). Consequently, no infection-driven effect on NPC1L1 gene transcription was assumed.
Treatments does not cause cytotoxic damage to host cells or tachyzoites
To evaluate if ezetimibe (20 μ) treatment induced tachyzoite dead trypan blue exclusion test was performed. Our data showed an average viability of 95.2% ± 1.3, 90.7% ± 1.9 and 93.7% ± 2.1 for T. gondii, N. caninum and B. besnoiti tachyzoites treated for 1 h with vehicle control (DMSO 0.06%) without significant effects provoked by ezetimibe (Fig. S1 A−C). Moreover, the cytotoxicity of ezetimibe or ezetimibe-glucuronide on endothelial host cells XTT test was performed. As illustrated in Fig. S1 D, treatments with ezetimibe or ezetimibe-glucuronide did not induce significant colorimetric changes in the formazan product compared to the vehicle control (DMSO 0.06%).
Discussion
Cholesterol is a major component of eukaryotic cell membranes (Luo et al., Reference Luo, Yang and Song2020). Given that apicomplexan parasites are generally considered as defective in cholesterol synthesis, they need to obtain this molecule from the host cell. Thus, LDL-driven cholesterol uptake is considered as a key pathway to fulfil cholesterol requirements during parasite merogony in different parasite species (Labaied et al., Reference Labaied, Jayabalasingham, Bano, Cha, Sandoval, Guan and Coppens2011; Coppens, Reference Coppens2013; Ehrenman et al., Reference Ehrenman, Wanyiri, Bhat, Ward and Coppens2013; Hamid et al., Reference Hamid, Hirzmann, Hermosilla and Taubert2014; Hamid et al., Reference Hamid, Hirzmann, Kerner, Gimpl, Lochnit, Hermosilla and Taubert2015; Nolan et al., Reference Nolan, Romano, Luechtefeld and Coppens2015; Taubert et al., Reference Taubert, Silva, Velásquez, Larrazabal, Lütjohann and Hermosilla2018; Silva et al., Reference Silva, Lutjohann, Hamid, Velasquez, Kerner, Larrazabal, Failing, Hermosilla and Taubert2019). Additionally, in case of C. parvum-infected Caco-2 cells, cholesterol is incorporated via NPC1L1-mediated micellar uptake (Ehrenman et al., Reference Ehrenman, Wanyiri, Bhat, Ward and Coppens2013). NPC1L1 is a trans-membrane protein highly expressed in enterocytes that mediates sterol internalization via clathrin-coated vesicles and it has widely been accepted as main target of the lipid-lowering drug ezetimibe (Altmann et al., Reference Altmann, Davis, Zhu, Yao, Hoos, Tetzloff, Iyer, Maguire, Golovko, Zeng, Wang, Murgolo and Graziano2004; Garcia-Calvo et al., Reference Garcia-Calvo, Lisnock, Bull, Hawes, Burnett, Braun, Crona, Davis, Dean, Detmers, Graziano, Hughes, Macintyre, Ogawa, O'Neill K, Iyer, Shevell, Smith, Tang, Makarewicz, Ujjainwalla, Altmann, Chapman and Thornberry2005; Betters and Yu, Reference Betters and Yu2010).
Current data demonstrate for the first time that ezetimibe has inhibitory effects on T. gondii, N. caninum, and B. besnoiti tachyzoite replication in primary host endothelial cells, i.e. a host cell type that is parasitized in vivo during the acute phase of toxoplasmosis, neosporosis and besnoitiosis (Maley et al., Reference Maley, Buxton, Rae, Wright, Schock, Bartley, Esteban-Redondo, Swales, Hamilton, Sales and Innes2003; Alvarez-Garcia et al., Reference Alvarez-Garcia, Frey, Mora and Schares2013; Konradt et al., Reference Konradt, Ueno, Christian, Delong, Pritchard, Herz, Bzik, Koshy, McGavern, Lodoen and Hunter2016). Here, 10 μ m ezetimibe treatment effectively blocked T. gondii and B. besnoiti proliferation, while N. caninum revealed less sensitive and inhibition needed a higher concentration of 20 μ m. However, both concentrations are in range or even lie below the concentration known to block effectively NPC1L1 endocytosis (25–100 μ m, Ehrenman et al., Reference Ehrenman, Wanyiri, Bhat, Ward and Coppens2013). Applying 20 μ m ezetimibe as effective concentration to all species studied, the anti-parasitic effects of this compound over time were explored. Its inhibitory effect on tachyzoite proliferation was consistent over time, since the number of newly released tachyzoites at 48, 72 and 96 h p. i. was consistently low. Thus, an overall reduction of tachyzoite production of 95.7, 73.1 and 99.2% was obtained for T. gondii, N. caninum and B. besnoiti, respectively. These findings in principle match data from C. parvum-infected Caco-2 cells (permanent cell line), where a growth reduction of 65% was achieved at 25–100 μ m concentrations (Ehrenman et al., Reference Ehrenman, Wanyiri, Bhat, Ward and Coppens2013). In vitro anti-parasitic efficacy of ezetimibe was also reported for L. amazonensis where 10 μ m ezetimibe reduced promastigote replication, however, amastigote production was only affected at double doses (20 μ m; Andrade-Neto et al., Reference Andrade-Neto, Cunha-Junior, Canto-Cavalheiro, Atella, Fernandes, Costa and Torres-Santos2016). This stage-specific discrepancy might be explained by a lower sensitivity of intracellular stages to ezetimibe treatments. Still, the concentrations here used do not necessarily support this assumption, since high effects were found at 10 μ m ezetimibe in case of T. gondii and B. besnoiti-infected BUVEC. Thus, stage- and species-related or even host cell type-related sensitivities may play a role. In line, P. falciparum-merozoite replication was effectively blocked only at much higher concentrations of 80 μ m ezetimibe in vitro (Hayakawa et al., Reference Hayakawa, Yamaguchi, Mori and Nardone2020).
Successful parasite offspring formation relies on several defined processes starting with active cell invasion, formation of the PV, replication and egress (Black and Boothroyd, Reference Black and Boothroyd2000). In this context, ezetimibe pre-treated extracellular tachyzoites showed impaired infection capacities, thereby hampering the replication process at the starting point. Of note, B. besnoiti tachyzoites appeared more sensitive to this treatment than T. gondii and N. caninum tachyzoites, the latter of which were hardly affected in their host cell invasion capacity.
A more detailed analysis of parasite intracellular development revealed an altered morphology of meronts in the case of all three parasites in treated host cells, suggesting that prolonged ezetimibe exposition indeed affected tachyzoite physiology, thereby reducing or hampering their ability to proliferate. Residual effects of ezetimibe on in vitro tachyzoite replication were evaluated by drug-withdrawal experiments. Notably, T. gondii and N. caninum tachyzoites recovered within 24 h post withdrawal and proliferated at normal replication rates thereby showing that ezetimibe mainly induced developmental arrest but did not kill the parasites. In contrast, B. besnoit tachyzoites suffered more profoundly from ezetimibe treatments since drug removal did not result in full recovery of parasite replication. These reactions indeed indicated species-specific sensitivity towards ezetimibe.
Overall, it is challenging to dissect if ezetimibe exclusively affects the parasites and/or the host cells, or if cumulative effects are to be considered. However, the current data showed that pre-treatments of host cells did not affect infection permissiveness since infection rates were similar in treated and control cells, when using non-treated tachyzoites for infection. Nevertheless, ezetimibe treatment of tachyzoites before host cell invasion led to a significant reduction of infection rates, suggesting that ezetimibe also directly acts on tachyzoite invasive capacity in a rather species-dependent manner. Besides this mode of action, anti-parasitic activity of ezetimibe was also associated with inhibition of parasite replication within PV. By using live cell 3D holotomographic microscopy as a reliable tool for 3D cell visualization in vivo (Silva et al., Reference Silva, Lutjohann, Hamid, Velasquez, Kerner, Larrazabal, Failing, Hermosilla and Taubert2019; Velásquez et al., Reference Velásquez, Conejeros, Larrazabal, Kerner, Hermosilla and Taubert2019), we showed that ezetimibe-treated host cells infected with T. gondii, N. caninum and B. besnoiti presented a reduced number of tachyzoites per meront. Summarizing these data, ezetimibe might act on both, extra- and intracellular tachyzoites.
Hypolipidaemic/cholesterol-lowering properties of ezetimibe have previously been reported for humans as well as animals (Bays et al., Reference Bays, Moore, Drehobl, Rosenblatt, Toth, Dujovne, Knopp, Lipka, Lebeaut, Yang, Mellars, Cuffie-Jackson, Veltri and Ezetimibe Study2001; van Heek et al., Reference van Heek, Farley, Compton, Hoos and Davis2001; Knopp et al., Reference Knopp, Gitter, Truitt, Bays, Manion, Lipka, LeBeaut, Suresh, Yang, Veltri and Ezetimibe Study2003). In vivo, this compound undergoes phase II metabolism to form a glucuronide conjugate, thereby improving NPC1L1-specific affinity and binding capacities (Garcia-Calvo et al., Reference Garcia-Calvo, Lisnock, Bull, Hawes, Burnett, Braun, Crona, Davis, Dean, Detmers, Graziano, Hughes, Macintyre, Ogawa, O'Neill K, Iyer, Shevell, Smith, Tang, Makarewicz, Ujjainwalla, Altmann, Chapman and Thornberry2005). Besides being the major metabolite detected in plasma (Garcia-Calvo et al., Reference Garcia-Calvo, Lisnock, Bull, Hawes, Burnett, Braun, Crona, Davis, Dean, Detmers, Graziano, Hughes, Macintyre, Ogawa, O'Neill K, Iyer, Shevell, Smith, Tang, Makarewicz, Ujjainwalla, Altmann, Chapman and Thornberry2005), ezetimibe-glucuronide is therefore considered as main active metabolite in ezetimibe treatments in vivo. To parallel in vivo situation, additional studies on the effect of ezetimibe-glucuronide on T. gondii, B. besnoiti and N. caninum tachyzoite proliferation in vitro were performed. Unexpectedly, treatments with ezetimibe-glucuronide failed to hamper intracellular tachyzoite replication thereby implicating that ezetimibe-mediated anti-parasitic effects might be NPC1L1-independent. In line, we were not able to demonstrate a consistent infection-driven induction of NPC1L1 mRNAs since these gene transcripts could hardly be detected in infected BUVEC or control cells even though intestinal control tissues gave good PCR signals. Consequently, we here assume a very low expression of this transporter in BUVEC. Likewise, the presence of NPC1L1 protein expression by Western blotting was not achieved, so far (unpublished data). Noteworthy, ezetimibe was originally identified as an ACAT II inhibitor (Clader, Reference Clader2004) and therefore as acting on other potential targets besides NPC1L1. Irrespective of this, it is well documented that NPC1L1 incorporates cholesterol through an ezetimibe-sensitive pathway, however, the binding mechanism between ezetimibe and NPC1L1 remains unknown (Betters and Yu, Reference Betters and Yu2010). Recently, it has been reported that ezetimibe, but not ezetimibe-glucuronide, reduces the cellular content of cholesteryl esters in a NPC1L1-independent manner in human monocytes, implying an inhibition of ACAT II (Orso et al., Reference Orso, Robenek, Boettcher, Wolf, Liebisch, Kramer and Schmitz2019). Likewise, we here propose that the current ezetimibe-mediated anti-coccidian effects may rather be linked to an inhibition of cholesterol esterification. In agreement, the importance of functional cholesterol esterification for coccidian replication was already confirmed for T. gondii (Sonda et al., Reference Sonda, Ting, Novak, Kim, Maher, Farese and Ernst2001), B. besnoiti (Silva et al., Reference Silva, Lutjohann, Hamid, Velasquez, Kerner, Larrazabal, Failing, Hermosilla and Taubert2019) and E. bovis (Hamid et al., Reference Hamid, Hirzmann, Hermosilla and Taubert2014). However, the actual role of ezetimibe in cholesterol esterification and its cytosolic targets in primary bovine endothelial host cells should be further addressed in future studies. Even though in vitro studies have been published reporting anti-parasitic activities of ezetimibe treatments, in vivo evidence still needs to be addressed. Nevertheless, administration of ezetimibe to L. amazonensis-infected mice led to reduced parasite burden and boosted anti-leishmanial activity of ketoconazole (Andrade-Neto et al., Reference Andrade-Neto, Cunha-Junior, Canto-Cavalheiro, Atella, Fernandes, Costa and Torres-Santos2016). However, given that ezetimibe treatments of P. yoelii-infected mice failed to affect parasitaemia (Kume et al., Reference Kume, Herbas, Shichiri, Ishida and Suzuki2016) phylum-derived differences have to be assumed.
In conclusion, the current study shows that ezetimibe effectively inhibits T. gondii, N. caninum and B. besnoiti tachyzoite replication in BUVEC in a time-sustained but reversible manner. Apparently, the anti-coccidian effect of ezetimibe is associated with both impairment of tachyzoite infectivity and intracellular replication blockage. Of note, we additionally observed that ezetimibe-glucuronide does not interfere with parasite replication thereby suggesting an NPC1L1-independent anti-parasitic mechanism.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182021000822.
Data
All data are available in the manuscript and Supplementary data files.
Acknowledgements
The authors would like to thank Christine Henrich, Dr Christin Ritter and Hannah Salecker for their outstanding technical support. We also are very thankful to Prof. Dr A. Wehrend (Clinic for Obstetrics, Gynaecology and Andrology of Large and Small Animals, Justus Liebig University, Giessen, Germany) for the continuous supply of bovine umbilical cords. Further, we thank Oliver Bender (Ubl butcher shop, Langsdorf, Germany) for the supply of bovine small intestine samples.
Author contributions
AT, CH, CL and LS conceived and designed the experiments. CL and LS performed the experiments. All authors performed analyses and interpretation of the data, preparation of the manuscript and approved final version of manuscript.
Financial support
CL was funded by the National Agency for Research and Development [(ANID), DOCTORADO BECAS CHILE/2017–72180349].
Conflict of interest
The authors declare there are no conflicts of interest.
Ethical standards
Not applicable