Introduction
Aquaglyceroporins (AQPs) were first identified in the 1990s as membrane proteins with functions in osmoregulation and the translocation of low molecular weight solutes, including glycerol and urea (Preston et al., Reference Preston, Carroll, Guggino and Agre1992). In humans, dysfunction is associated with multiple cancers, kidney disease, oedema and other pathologies (King et al., Reference King, Kozono and Agre2004; Yool et al., Reference Yool, Brown and Flynn2010; Shi et al., Reference Shi, Zhang, Luo, Zhao, Cheng, Xiang and Zhao2012). AQPs have an evolutionarily broad representation, being found in most pro- and eukaryotic taxa and they retain a conserved architecture encompassing six hydrophobic domains. This structure is in turn derived through an internal duplication from a primordial protein with three membrane-spanning helices, reflected in the presence of two NPA (Asn-Pro-Ala) boxes that are involved in channel functions. Both the N- and C-termini face the cytoplasm (Fig. 1) and sequence and architectural conservation indicates vertical descent. Consequently, at least one mechanism for the control of water (and solute) passage across biological membranes arose very early in the history of life (Ishibashi et al., Reference Ishibashi, Tanaka and Morishita2020). However, AQPs are not present in all taxa, for example the bacterial phyla Fibrobacteres and Lentisphaerae, as well as some parasites and extremophiles. As AQPs can also be deleted in some eukaryotes, for example immortalized mammalian cells and trypanosomatids (Jeacock et al., Reference Jeacock, Baker, Wiedemar, Mäser and Horn2017; Calvanese et al., Reference Calvanese, D'Auria, Vangone, Falcigno and Oliva2018), it is clear that AQPs are non-essential, at least under some circumstances. Control of osmolarity therefore likely utilizes additional mechanisms in both pro- and eukaryotes. Below we will consider initially the evolution and origins of AQP paralogues in protists and then the uncovering of drug-related functions in trypanosomes.
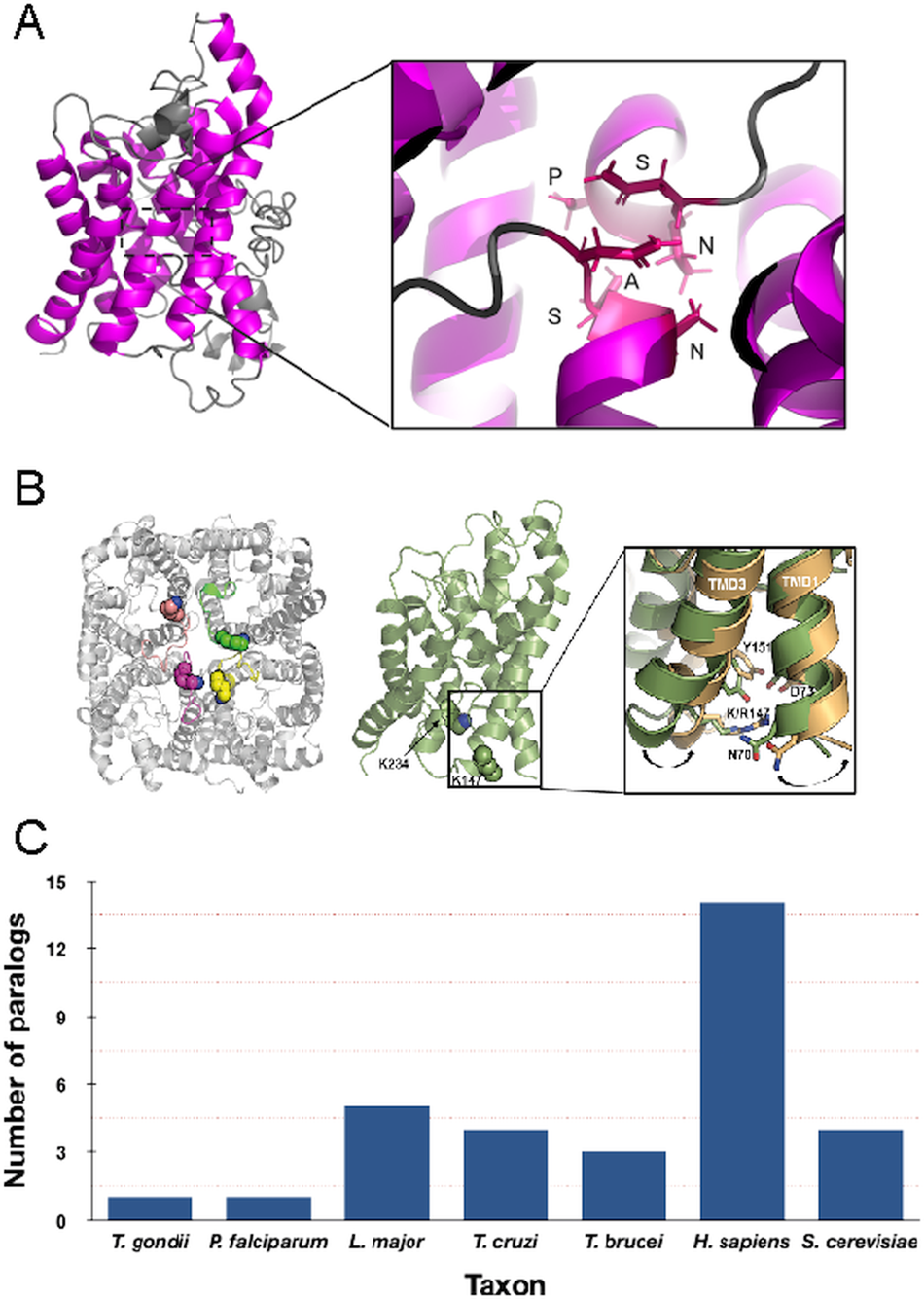
Fig. 1. Structure and copy number of AQP paralogues. (A) Left panel: Depiction of the Trypanosoma brucei AQP2 monomer. The trans-membrane domains are highlighted in magenta. Right panel: Details of the unique NPS/NSA TbAQP2 selectivity pore. (B) Left panel: Top view of the proposed tetrameric structure of T. brucei AQP2 model. The lysine residues in position K147 and K234 are shown as spheres. Right panel: Expanded view of the conformational change observed during TMD simulations on TMD1 and TMD3 as a result of the K147R mutation. Wild type TbAQP2 is shown in green. TbAQP2 displaying the K147R and K234R mutations is shown in light orange. Other residues important for intramolecular interactions between transmembrane domains (N70, D73, K142 and Y151) are also highlighted. Mutations on these residues profoundly impair protein stability, rendering the parasites resistant to pentamidine and melarsoprol. (Quintana et al., Reference Quintana, Bueren-Calabuig, Zuccotto, De Koning, Horn and Field2020). (C) Number of clear AQP paralogues detected in representative taxa. Note that for the protists these are all represented by the more permissive glycerol-capable class.
Evolution, functions and roles in protists
The evolution of the AQP family is surprisingly complex and at least three subfamilies with apparently distinct functions are recognized. These include AQPs able to translocate glycerol, others that only uptake water and a final third group, the superAQPs, that arose late in evolution. This latter subfamily is frequently intracellular, indicating a distinct function from the other members of the AQP family, which are usually located at the surface in most cells (Ishibashi et al., Reference Ishibashi, Kondo, Hara and Morishita2011), and are only found in metazoa. Significantly, the two ancestral forms are clearly differentiated in all prokaryotes, indicating an origin dating back to an early period of cellular life (Tong et al., Reference Tong, Hu, Zhu and Dong2019). The number of AQP paralogues in different species is highly variable, with land plants and vertebrates having the largest repertoires, as is the case for many other protein families.
There has been a considerable degree of expansion and contraction within specific lineages, or ‘churning’, with the result that functional differentiation between paralogues is difficult to predict (Ishibashi et al., Reference Ishibashi, Tanaka and Morishita2020). Interestingly, in mussels (molluscs) there is evidence that expansion of AQP paralogues correlates with freshwater colonization events and hence facilitating adaptation to decreased environmental salinity (Calcino et al., Reference Calcino, De Oliveira, Simakov, Schwaha, Zieger, Wollesen and Wanninger2019). Similar events may have facilitated tetrapod colonization of land habitats where desiccation is a considerable challenge (Finn et al., Reference Finn, Chauvigné, Hlidberg, Cutler and Cerdà2014) and underscores the importance of AQP evolution to life history.
In unicellular eukaryotes the number of AQP paralogues is comparatively small when compared with multicellular organisms and it has been proposed that the numbers of AQP paralogues are correlated somewhat with environmental complexity (von Bülow and Beitz, Reference von Bülow and Beitz2015). Most protist AQPs appear to be the more permissive glycerol-translocating forms that facilitate the uptake of solutes and waste compounds in addition to water. The free-living amoeba Amoeba proteus expresses a single AQP that is associated with the contractile vacuole (Nishihara et al., Reference Nishihara, Yokota, Tazaki, Orii, Katsuhara, Kataoka, Igarashi, Moriyama and Seiji2012), but by contrast there are four AQP paralogues in the social amoeba Dictyostelium discoideum, two of which are constitutively expressed and the remainder stage specific. Although there is evidence for roles in differentiation, none of the D. discoideum AQPs are exclusively water permeable and hence functions are not completely clear (Von Bülow et al., Reference Von Bülow, Müller-Lucks, Kais, Bernhards and Beitz2012). In the parasites Plasmodium falciparum and Toxoplasma gondii, each have a single AQP (Fig. 2) and this minimal repertoire may reflect intracellular life cycles and a more constant environment, albeit with considerable levels of complexity and differentiation events during life cycle progression, particularly for P. falciparum.

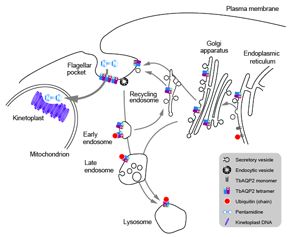
Fig. 2. TbAQP2 trafficking, assembly and pentamidine uptake. Schematic of the trypanosome endomembrane system, focused on the region between the nucleus and flagellar pocket and encompassing the mitochondrion. AQP proteins are represented as open coloured cylinders, with the opening indicting the cell external/intracellular luminal face of the molecule. Note that both ER and endosomal molecules can become ubiquitylated (red dot). It is most likely that pentamidine enters the cell at the cell surface (see text) and is then translocated into the mitochondrion to interact with the kinetoplast (mitochondrial genome, purple circles) but he possibility that there is a contribution from endocytosis of AQP; pentamidine complexes remains a possibility.
Amongst the kinetoplastids, Leishmania major has five AQPs, although only AQP1 has been studied in any detail. LmAQP1 is a wide permeability form localized on the flagellum and regulated by MAP kinase (Figarella et al., Reference Figarella, Uzcátegui, Zhou, LeFurgey, Ouellette, Bhattacharjee and Mukkhopadhyay2007; Mandal et al., Reference Mandal, Sharma, Kruse, Sander-Juelch, Munro, Wang, Vilg, Tamás, Bhattacharjee, Wiese and Mukhopadhyay2012; Sharma et al., Reference Sharma, Mandal, Mandal and Bhattacharjee2015). The remaining L. major AQPs are less well uncharacterized, but at the sequence level more closely resemble the plant tonoplast intrinsic protein (TIP) AQP subclass. Four of the five AQP genes in L. donovani retain canonical gating motifs, but in one paralogue this is mutated to NPM-NPA. All four of the conventional AQPs are suggested as intracellular as is the case for the TIP AQPs of plants, but significantly LdAQP1 is likely to permit translocation of large solutes (Biyani et al., Reference Biyani, Mandal, Seth, Saint, Natarajan, Ghosh and Madhubala2011). Antimonial-containing drugs remain a first line treatment against Leishmania in many parts of the world (Field et al., Reference Field, Horn, Fairlamb, Ferguson, Gray, Read, De Rycker, Torrie, Wyatt, Wyllie and Gilbert2017) and in laboratory derived strains of Leishmania mexicana AQP1 can restore antimonial uptake to resistant cells (Marquis et al., Reference Marquis, Gourbal, Rosen, Mukhopadhyay and Ouellette2005). No obvious genome level changes to gene copy number or sequence accompany resistance but is potentially a post-transcriptional modulation of AQP1 mRNA level. Changes to expression of AQP1 have been demonstrated in multiple species where resistance was derived in the laboratory (Lin et al., Reference Lin, Hsu, Shu, Chi, Chiang and Lee2008; Barrera et al., Reference Barrera, Rojas, Weiss, Fernandez, McMahon-Pratt, Saravia and Gomez2017). However, it is also clear that there is a less compelling case for association of altered AQP1 expression and drug resistance in clinical isolates.
The American trypanosome, Trypanosoma cruzi also has four TIP-like AQPs, representing the entire repertoire in that organism and these are associated with the contractile vacuole and acidocalcisomes (Montalvetti et al., Reference Montalvetti, Rohloff and Docampo2004). Trypanosoma brucei has three AQPs; AQP1 is shared with other kinetoplastida, while AQP2 and AQP3 arose from a recent gene duplication in the African trypanosome lineage and remain contiguous.
In addition to interactions between trans-membrane domains, two major selectivity filters restrict the molecular weights and properties of the solutes being translocated by AQPs and that can effectively pass through the central pore; these are the ar/R and NPA/NPA motifs (Fig. 1) (Beitz, Reference Beitz2005; Baker et al., Reference Baker, De Koning, Mäser and Horn2013; Verkman et al., Reference Verkman, Anderson and Papadopoulos2014; Munday et al., Reference Munday, Settimo and de Koning2015; Fairlamb and Horn, Reference Fairlamb and Horn2018). Trypanosoma brucei AQP1 and AQP3 display the internal arrangements in the protein pore observed in canonical AQPs, including the canonical ‘NPA’ within two half α-helices and a narrower ‘aromatic/arginine’ (ar/R) motif (Beitz, Reference Beitz2005). Interestingly, TbAQP2 does not retain this canonical configuration, displaying an unconventional ‘NPS/NSA’ filter motif and rearrangement in the ar/R motif that is replaced by a neutral leucine at position 264 (L264), followed by aliphatic, rather than aromatic, residues (A88, I110, V249 and L258), which are equivalent to the ‘IVLL’ motif observed in the selectivity pore of canonical AQPs (de Groot and Grubmuller, Reference de Groot and Grubmuller2001; Baker et al., Reference Baker, De Koning, Mäser and Horn2013; Quintana et al., Reference Quintana, Bueren-Calabuig, Zuccotto, de Koning, Horn and Field2020). These structural features indicate that TbAQP2 can accommodate larger solutes through the selectivity pore (Uzcategui et al., Reference Uzcategui, Szallies, Pavlovic-Djuranovic, Palmada, Figarella, Boehmer, Lang, Beitz and Duszenko2004).
These examples demonstrated that AQP evolution is highly plastic, with the creation of additional paralogues, facilitating altered specificity. Hence, the AQP family contributes to surviving environmental complexity and exploitation of new ecological niches, with a considerable impact on the life history of the earth. However, the absence of AQPs from many lineages or a genetic demonstration of essentially in many organisms serves to underscore the challenges remaining for the full understanding of AQP function.
TbAQP2 and multidrug resistance
The treatment of sleeping sickness relies on drugs to clear first- or second-stage infections, and the choice of drug depends on the capacity to penetrate the blood–brain barrier (BBB) (Denise and Barrett, Reference Denise and Barrett2001; Steverding, Reference Steverding2010; Fairlamb and Horn, Reference Fairlamb and Horn2018). Of these, pentamidine and melarsoprol represent two of the most potent drugs currently used to treat first- and second-stage diseases, respectively, displaying low nanomolar 50%-effective growth-inhibitory concentration (EC50) (Denise and Barrett, Reference Denise and Barrett2001; Bray et al., Reference Bray, Barrett, Ward and De Koning2003; Barrett et al., Reference Barrett, Boykin, Brun and Tidwell2007; Fairlamb and Horn, Reference Fairlamb and Horn2018). Pentamidine, an aromatic diamidine, is used to treat first-stage (haemolymphatic stage) T. gambiense HAT (Denise and Barrett, Reference Denise and Barrett2001; Barrett et al., Reference Barrett, Boykin, Brun and Tidwell2007; Baker et al., Reference Baker, De Koning, Mäser and Horn2013). This compound binds nucleic acids with high affinity, leading to accumulation by, and ultimately destruction of, the kinetoplast (Mathis et al., Reference Mathis, Holman, Sturk, Ismail, Boykin, Tidwell and Hall2006; Baker et al., Reference Baker, De Koning, Mäser and Horn2013; Gould and Schnaufer, Reference Gould and Schnaufer2014; Al-Horani et al., Reference Al-Horani, Clemons and Mottamal2019; Kennedy and Rodgers, Reference Kennedy and Rodgers2019). However, pentamidine is unable to reach the central nervous system (CNS), in part due to its high affinity interactions with serum proteins, charge and relatively high retention in tissues and is therefore ineffective for the treatment of second-stage meningoencephalic HAT (Barrett et al., Reference Barrett, Boykin, Brun and Tidwell2007; Maclean et al., Reference Maclean, Reiber, Kennedy and Sternberg2012). Melarsoprol, on the contrary, is an arsenical compound used for the treatment of second-stage HAT, including T. rhodesiense HAT (Fairlamb et al., Reference Fairlamb, Henderson and Cerami1989; Keiser et al., Reference Keiser, Ericsson and Burri2000; Field et al., Reference Field, Horn, Fairlamb, Ferguson, Gray, Read, De Rycker, Torrie, Wyatt, Wyllie and Gilbert2017). This compound is thought to be metabolized to melarsen oxide prior to uptake by African trypanosomes, leading to the formation of a stable adduct with trypanothione known as Mel T (Burri et al., Reference Burri, Baltz, Giroud, Doua, Welker and Brun1993, Reference Burri, Onyango, Auma, Burudi and Brun1994; Fairlamb and Horn, Reference Fairlamb and Horn2018). Melarsoprol penetrates the BBB comparatively more effectively than pentamidine, reaching the minimum concentration required for parasite clearance in the CNS (Mäser et al., Reference Mäser, Sütterlin, Kralli and Kaminsky1999; Stewart et al., Reference Stewart, Burchmore, Clucas, Hertz-Fowler, Brooks, Tait, MacLeod, Turner, de Koning, Wong and Barrett2010). Melasoprol also displays reactive encephalopathy in ~10% of patients, which is frequently fatal (Fairlamb and Horn, Reference Fairlamb and Horn2018).
Given the limited repertoire of drugs available for treatment it is perhaps not surprising that resistance to these compounds has been frequently observed in endemic countries. Indeed, diamidine-arsenical cross-resistance was initially reported in the 1940s, suggesting that mechanisms of uptake and/or action were common to these otherwise divergent chemical compounds, but with the molecular details poorly understood. The identification of the pentamidine/melarsoprol transporter has been a serendipitous process. Initial studies in cross-resistance in laboratory strains (Bernhard et al., Reference Bernhard, Nerima, Mäser and Brun2007; Bridges et al., Reference Bridges, Gould, Nerima, Mäser, Burchmore and de2007; Graf et al., Reference Graf, Baker, Munday, de Koning, Horn and Mäser2015a) and field isolates (Shahi et al., Reference Shahi, Krauth-Siegel and Clayton2002; Alsford et al., Reference Alsford, Eckert, Baker, Glover, Sanchez-Flores, Leung, Turner, Field, Berriman and Horn2012) from relapsed patients identified the gene encoding for the purine transporter responsible for drug uptake as T. brucei adenosine transporter 1 (TbAT1). In addition to TbAT1, the high-affinity pentamidine transporter (HAPT1) (Bernhard et al., Reference Bernhard, Nerima, Mäser and Brun2007) as well as the ATP-binding cassette transporter MRPA (Baker et al., Reference Baker, Glover, Munday, Aguinaga Andres, Barrett, de Koning and Horn2012) were also proposed to mediate drug resistance by various mechanisms, but neither explained the drug resistance levels observed in field isolates (Baker et al., Reference Baker, De Koning, Mäser and Horn2013).
Using genome-wide RNAi-mediated genetic screening and functional assays, the locus encoding the closely related AQP2 and AQP3 was identified as a bona fide hit for pentamidine/melarsoprol cross-resistance (Graf et al., Reference Graf, Baker, Munday, De Koning, Horn and Mäser2015b). Further biochemical and genetic manipulation studies demonstrated that deletion of AQP2, but not AQP3, led to a significant increase in the EC50 of both compounds, mirroring the behaviour observed in previously generated laboratory strains and field isolates (Munday et al., Reference Munday, Eze, Baker, Glover, Clucas, Andrés, Natto, Teka, Mcdonald, Lee, Graf, Ludin, Burchmore, Turner, Tait, Macleod, Mäser, Barrett, Horn and De Koning2014; Graf et al., Reference Graf, Baker, Munday, De Koning, Horn and Mäser2015b; Song et al., Reference Song, Baker, Rothert, Henke, Jeacock, Horn and Beitz2016). Other observations such as localization to the flagellar pocket in the bloodstream form (Munday et al., Reference Munday, Eze, Baker, Glover, Clucas, Andrés, Natto, Teka, Mcdonald, Lee, Graf, Ludin, Burchmore, Turner, Tait, Macleod, Mäser, Barrett, Horn and De Koning2014; Graf et al., Reference Graf, Baker, Munday, De Koning, Horn and Mäser2015b; Song et al., Reference Song, Baker, Rothert, Henke, Jeacock, Horn and Beitz2016; Quintana et al., Reference Quintana, Bueren-Calabuig, Zuccotto, de Koning, Horn and Field2020), as well as the unusual pore structure discussed above, led to the hypothesis that pentamidine and melarsoprol are likely to interact with high affinity to AQP2 located in the flagellar pocket (Alghamdi et al., Reference Alghamdi, Munday, Campagnaro, Gurvic, Svensson, Okpara, Kumar, Quintana, Abril, Milić, Watson, Paape, Settimo, Dimitriou, Wielinska, Smart, Anderson, Woodley, Kelly, Ibrahim, Hulpia, Al-Salabi, Eze, Sprenger, Teka, Gudin, Weyand, Field, Dardonville, Tidwell, Carrington, O'neill, Boykin, Zachariae and De Koning2020), posing the question of how these compounds are internalized and also the mechanisms for resistance.
Endocytosis or membrane uptake: competing models for drug entry
Suggesting that the role of a channel protein is not the primary mechanism for pentamidine to access the trypanosome cytoplasm may seem to be a straw man, but this possibility has been proposed. Specifically, as AQP2 binds pentamidine with high affinity at the first selectivity pore, the possibility that AQP2 is a receptor for uptake by endocytosis is not unreasonable (Fig. 2) and could act as a parallel to ISG75-mediated uptake of suramin (Graf et al., Reference Graf, Baker, Munday, De Koning, Horn and Mäser2015b). This model was further supported by reports demonstrating that pentamidine binds AQP2 with nanomolar affinity, thus potentially acting as a highly selective inhibitor of AQP2 (Fig. 2) (Alghamdi et al., Reference Alghamdi, Munday, Campagnaro, Gurvic, Svensson, Okpara, Kumar, Quintana, Abril, Milić, Watson, Paape, Settimo, Dimitriou, Wielinska, Smart, Anderson, Woodley, Kelly, Ibrahim, Hulpia, Al-Salabi, Eze, Sprenger, Teka, Gudin, Weyand, Field, Dardonville, Tidwell, Carrington, O'neill, Boykin, Zachariae and De Koning2020). However, consideration of structural features of the pore do support TbAQP2 acting as a channel for larger and more structurally flexible solutes including pentamidine (Petersen and Beitz, Reference Petersen and Beitz2020). In the endocytosis model, ubiquitination of TbAQP2 at the flagellar pocket is central for subsequent ubiquitination-mediated intracellular trafficking and delivery to intracellular organelles such as the lysosome. Indeed, TbAQP2 forms a stable homomultimeric complex in the flagellar pocket where ubiquitination is likely to take place on individual monomers (Quintana et al., Reference Quintana, Bueren-Calabuig, Zuccotto, de Koning, Horn and Field2020).
The opposing membrane uptake model proposes that pentamidine, and potentially melarsoprol, are taken up via the intrinsic channel properties of TbAQP2. Indeed, a recent report demonstrates that drug permeation is possible due to a highly conserved amino acid motif in the central pore architecture of TbAQP2, facilitating the passage of ‘high’ molecular weight solutes (Alghamdi et al., Reference Alghamdi, Munday, Campagnaro, Gurvic, Svensson, Okpara, Kumar, Quintana, Abril, Milić, Watson, Paape, Settimo, Dimitriou, Wielinska, Smart, Anderson, Woodley, Kelly, Ibrahim, Hulpia, Al-Salabi, Eze, Sprenger, Teka, Gudin, Weyand, Field, Dardonville, Tidwell, Carrington, O'neill, Boykin, Zachariae and De Koning2020). This was demonstrated by TbAQP3 mutants containing the amino acids of the selectivity pore from TbAQP2 possessing increased capacity for pentamidine uptake (Alghamdi et al., Reference Alghamdi, Munday, Campagnaro, Gurvic, Svensson, Okpara, Kumar, Quintana, Abril, Milić, Watson, Paape, Settimo, Dimitriou, Wielinska, Smart, Anderson, Woodley, Kelly, Ibrahim, Hulpia, Al-Salabi, Eze, Sprenger, Teka, Gudin, Weyand, Field, Dardonville, Tidwell, Carrington, O'neill, Boykin, Zachariae and De Koning2020). Moreover, pentamidine permeation through TbAQP2 seems to be further aided by the intrinsic membrane potential and is not abrogated by partially blocking endocytic uptake (Alghamdi et al., Reference Alghamdi, Munday, Campagnaro, Gurvic, Svensson, Okpara, Kumar, Quintana, Abril, Milić, Watson, Paape, Settimo, Dimitriou, Wielinska, Smart, Anderson, Woodley, Kelly, Ibrahim, Hulpia, Al-Salabi, Eze, Sprenger, Teka, Gudin, Weyand, Field, Dardonville, Tidwell, Carrington, O'neill, Boykin, Zachariae and De Koning2020; Quintana et al., Reference Quintana, Bueren-Calabuig, Zuccotto, de Koning, Horn and Field2020), albeit at a rate that is considerably slower than for lower molecular weight solutes, which in essence implies a leak in the AQP2 permeability barrier.
Concerning the likely site for pentamidine uptake, there is no evidence that endocytosis or post-translational modification of AQP2 is required. Specifically, additional genes identified from the genome-wide RNAi screen identified a kinase and phosphatase for melarsoprol and pentamidine respectively, as well as one unique hypothetical each (Alsford et al., Reference Alsford, Eckert, Baker, Glover, Sanchez-Flores, Leung, Turner, Field, Berriman and Horn2012). None of these genes have evidence for roles in ubiquitylation, endocytosis or trafficking in general, suggesting that translocation of drugs from the surface is sufficient for toxicity and that blocking ubiquitylation or endocytosis does not offer resistance. However, it needs to be acknowledged that a role for endocytosis that is overshadowed by the channel-mediated mechanism, remains a possibility.
Stability and folding of TbAQP2 contribute to pentamidine resistance
In common with most membrane proteins, AQPs undergoing translation are inserted into the endoplasmic reticulum through the Sec61 translocon and assisted in folding via a cohort of chaperones (Pitonzo and Skach, Reference Pitonzo and Skach2006). Given that most AQPs are also glycoproteins it is likely that the calnexin/calreticulin quality control system is involved in monitoring quality and rapidity of folding. Importantly, formation of homotetrameric complexes is important for AQP stability and the formation of heterotetrameric complexes has not been observed (Duchesne et al., Reference Duchesne, Pellerin, Delamarche, Deschamps, Lagrée, Froger, Bonnec, Thomas and Hubert2002; Furman et al., Reference Furman, Gorelick-feldman, Davidson, Yasumura, Neely, Agre and Rash2003). The residues responsible for this specificity are not clear, but AQP tetramers can assemble into higher order quasi-crystalline arrays (Kitchen et al., Reference Kitchen, Conner, Bill and Conner2016). Furthermore, there are clear differences in the stabilities of the water and solute permeable AQP tetramers with the former exhibiting greater stability than the latter and likely due to features within the final two trans-membrane domains and loops D and E (Lagrée et al., Reference Lagrée, Froger, Deschamps, Pellerin, Delamarche, Bonnec, Gouranton, Thomas and Hubert1998; Duchesne et al., Reference Duchesne, Pellerin, Delamarche, Deschamps, Lagrée, Froger, Bonnec, Thomas and Hubert2002; Buck et al., Reference Buck, Wagner, Grund and Skach2007; Kitchen et al., Reference Kitchen, Conner, Bill and Conner2016), albeit with the functional consequences, if any, unclear. Significantly the folding pathway is not identical for all AQPs, being controlled at least partly by sequences within the second trans-membrane domain (Carrington et al., Reference Carrington, Field, Sergeenko, Wang and Bo2010). Finally, mammalian AQPs are both phosphorylated and ubiquitylated, with at least the latter contributing to protein turnover, endocytosis and quality control (Kamsteeg et al., Reference Kamsteeg, Hendriks, Boone, Konings, Oorschot, van der Sluijs, Klumperman, Deen, Van Der Sluijs, Klumperman, Deen, van der Sluijs, Klumperman and Deen2006; Mandal et al., Reference Mandal, Sharma, Kruse, Sander-Juelch, Munro, Wang, Vilg, Tamás, Bhattacharjee, Wiese and Mukhopadhyay2012; Sharma et al., Reference Sharma, Mandal, Mandal and Bhattacharjee2015; Quintana et al., Reference Quintana, Bueren-Calabuig, Zuccotto, de Koning, Horn and Field2020). Although it is most likely that similar pathways operate in trypanosomes, with direct evidence for ubiquitylation and most of the relevant folding chaperones present, the precise mechanisms of AQP maturation, at least in African trypanosomes, remain to be investigated in detail (Field et al., Reference Field, Sergeenko, Wang, Böhm, Carrington, Field, Sergeenko, Wang and Bo2010; Tiengwe et al., Reference Tiengwe, Muratore and Bangs2016a, Reference Tiengwe, Muratore and Bangs2016b).
To understand folding, stability and trafficking of AQP2 in T. brucei we examined sequence-dependence and trans-membrane domain exchange designed to mimic natural AQP2/3 chimeras expressed in a triple null background (Jeacock et al., Reference Jeacock, Baker, Wiedemar, Mäser and Horn2017; Quintana et al., Reference Quintana, Bueren-Calabuig, Zuccotto, de Koning, Horn and Field2020). TbAQP2 forms both tetramers and tetramers of tetramers and is degraded in the lysosome by a ubiquitin-dependent process (Fig. 2) (Quintana et al., Reference Quintana, Bueren-Calabuig, Zuccotto, de Koning, Horn and Field2020). Attempts to influence ubiquitination by mutating cytoplasmic lysine residues unexpectedly reduce stability rather than preventing lysosomal targeting (Quintana et al., Reference Quintana, Bueren-Calabuig, Zuccotto, de Koning, Horn and Field2020). This is due to reduced folding and tetramerization efficiency, which triggers ER-associated degradation, indicating a failure to complete quality control (Quintana et al., Reference Quintana, Bueren-Calabuig, Zuccotto, de Koning, Horn and Field2020). Perhaps the most significant finding is that chimerical TbAQP2/3 proteins also lead to impaired folding and reduced stability (Quintana et al., Reference Quintana, Bueren-Calabuig, Zuccotto, de Koning, Horn and Field2020). This was also the case for constructs mimicking chimeras found in trypanosomes from patients where pentamidine treatment had failed.
Clearly rigorous quality control mechanisms operate within the ER of T. brucei, but with a consequence that mutations in the non-essential AQPs can render parasites refractory to treatment. Moreover, the instability of AQP2 is likely an underlying cause of pentamidine treatment failure while the production of chimeric forms is potentially a high frequency event and stems directly from generation of contiguous paralogues initially derived by gene duplication; presumably the chimeras have poor folding capability due to mismatch between the N- and C-terminal regions.
Concluding remarks
Remarkable advances to understanding mechanisms for classical therapies against African trypanosomes, as well as development of new drugs and the successes of public health programmes, auger well for the control of both human and animal African trypanosomiasis. Remarkably, we now have considerable understanding of pentamidine and melarsoprol uptake as well as mechanisms for resistance. The evolutionary history of trypanosome AQPs reveals both how pentamidine sensitivity arose, with a specifically broad-spectrum AQP2, and resistance arising from recombination. Placed in context (Fig. 3) the millennia-old relationship between trypanosomes and humans has been complex, with periods where one organism had the upper hand and then the other. Recently, humans have been in the ascendant, with case numbers having dropped precipitously and even exceeding the WHO roadmap predictions. Indeed, several countries previously considered endemic have reported no cases for several years. It can only be hoped that the advances made in the last decade are not eroded by the COVID-19 pandemic, which threatens to undermine global progress on many fronts, including the control of infectious diseases (http://hdrundp.org/en/2020-report).

Fig. 3. Major events in the history of African trypanosomiasis. Annotations above the timeline in black indicate major cultural, historical and management events with a bearing on trypanosomiasis. The influence on hominid evolution is inferred from impact on savannah ecosystems. Annotations in green indicate introduction of chemotherapeutic agents and in red emergence of resistance mechanisms and related advances in molecular understanding. Annotated beneath the timeline are periods of major change in the incidence of trypanosomiasis, in red for periods of epidemics and teal for control measures.
Financial support
Work in the Dundee laboratory is supported by the Wellcome Trust (204697/Z/16/Z). JFQ is supported by a Sir Henry Wellcome postdoctoral fellowship (221640/Z/20/Z).
Conflict of interest
None.