Introduction
The ‘increased host ability hypothesis’ posits that parasites manipulate the behaviour of their intermediate hosts in order to avoid predation by non-host species in order to enhance their transmission to the next host (Médoc and Beisel, Reference Medoc and Beisel2008). The life cycle of acanthocephalan parasites includes intermediate hosts, such as amphipods, isopods, ostracods, copepods, insects and myriapods (Nickol, Reference Nickol, Crompton and Nickol1985). The acanthocephalan parasite, Polymorphus minutus, appears capable of altering the behaviour of its intermediate hosts (amphipods) in a manner that enhances their transmission to the definitive host, the water fowl (Bakker et al., Reference Bakker, Mazzi and Zala1997; Kaldonski et al., Reference Kaldonski, Perrot-Minnot and Cézilly2007; Perrot-Minnot et al., Reference Perrot-Minnot, Kaldonski and Cézilly2007; Médoc and Beisel, Reference Medoc and Beisel2008).
Earlier studies have demonstrated that parasites decrease non-host predator exposure by manipulating their host's predator avoidance behaviour (Holomuzki et al., Reference Holomuzki, Short, Holomuzki, Short and Morgan1988; Rigby and Jokela, Reference Rigby and Jokela2000; Matz and Kjelleberg, Reference Matz and Kjelleberg2005; Friman et al., Reference Friman, Lindstedt, Hiltunen, Laakso and Mappes2009). Parasites modify the host's behaviour by regulating the host's neurotransmitter serotonin levels (Tain et al., Reference Tain, Perrot-Minnot and Cezilly2006; Cézilly and Perrot-Minnot, Reference Cézilly and Perrot-Minnot2010; Cézilly et al., Reference Cezilly, Favrat and Perrot-Minnot2013) or through the production of anaerobic metabolites (such as lactate and succinate; Perrot-Minnot et al., Reference Perrot-Minnot, Maddaleno and Cézilly2016). However, one fundamental question remains; has such ability to manipulate the host's behaviour evolved specifically to target the host genus in general, or only sympatric, co-existing host species.
The aim of this study was to assess if modulation of the host's behaviour by P. minutus is specific to sympatric gammarids or gammarids in general. Earlier studies suggested that modulation of the host behaviour evolved through ‘adaptation’ (Baldauf et al., Reference Baldauf, Thünken, Frommen, Bakker, Heupel and Kullmann2007) and ‘exaptation’ (Combes, Reference Combes2005; Beisel and Médoc, Reference Beisel and Médoc2010) by parasites or both (Cézilly and Perrot-Minnot, Reference Cézilly and Perrot-Minnot2005). Exaptation describes the case when a behavioural trait (e.g. negative geotaxis) in P. minutus, that evolved in response to predation, ultimately facilitates trophic transmission to the definitive host, i.e. due to an elevated level of avoidance towards non-host predators (Rohde, Reference Rohde1994; Beisel and Médoc, Reference Beisel and Médoc2010).
The native gammarids, Gammarus pulex, and, Gammarus fossarum, are abundant in Central European upper and middle freshwater tributaries from Danube to Rhine drainage systems (Siegismund and Müller, Reference Siegismund and Müller1991). In contrast, the gammarid Echinogammarus berilloni is native to the Atlantic regions of France and Spain (Médoc et al., Reference Médoc, Albert and Spataro2015), but is an invasive species in western and Central Europe (Schmidt-Drewello et al., Reference Schmidt-Drewello, Riss, Scharsack and Meyer2016). The gammarids are predated upon by water fowl, which are P. minutus' definitive hosts, but also by non-host predators, such as three-spined sticklebacks, Gasterosteus aculeatus (Médoc et al., Reference Médoc, Rigaud, Bollache and Beisel2009). The three-spined sticklebacks are native to the Paderborn Plateau in Westphalia, Germany (Schmidt-Drewello et al., Reference Schmidt-Drewello, Riss, Scharsack and Meyer2016). Consequently, avoiding predation of gammarid hosts by non-host predators would increase the probability of predation of gammarids by water fowl and hence their transmission to the definitive host.
The objective of this study was to assess if P. minutus infected gammarids altered the gammarids behaviour towards non-host predators, more specifically three-spined sticklebacks, and if such a change was observed in all, or only sympatric, gammarid species. We tested whether the ‘increased host ability hypothesis’ in non-host predators, was more pronounced in native, congenerics, sympatric co-evolved gammarids in comparison to sympatric invasive gammarids.
Materials and methods
Sampling
Specimens of G. pulex were collected in the Altenau river (51°35′56.73″N;8°46′56.92″E), whereas specimens of G. fossarum and E. berilloni were collected in the upper and lower parts of the Alme river, respectively (51°32′50.05″N; 8°32′43.61″E). Sampling took place during May and June in 2010. The prevalence of P. minutus in all three gammarids was below 1% in the Paderborn Plateau river system (Niehof and S. Farahani, unpublished data). Both river systems are also home to several fish species that prey on gammarids, such as three-spined sticklebacks (Schmidt-Drewello et al., Reference Schmidt-Drewello, Riss, Scharsack and Meyer2016), river trout Salmo trutta fario, minnow Phoxinus phoxinus, northern pike Esox Lucius, chub Squalius cephalus, rainbow trout Oncorhynchus mykiss, freshwater sculpin Cottus gobio and grayling Thymallus thymallus. Both tributaries are located on the Paderborn Plateau in Westphalia, Germany. All sampled gammarids were kept in Dewar flasks in water from the sampling site during transport (~90 min). On arrival in the laboratory sampled gammarids were transferred to aquaria (50 × 50 × 25 cm) with aerated tap water at 16 ± 1°C (water temperature at sampling site) and kept for two or three weeks. Infected and uninfected gammarids were kept in separate aquaria. Infected gammarids were identified from the number of orange-red spots (cystacanth, the developmental stage at which the parasite is able to infect its definitive hosts) present on the cuticle (Dezfuli and Giari, Reference Dezfuli and Giari1999). A dark:light cycle of 10:14 h was maintained in order to mimic seasonal conditions. Gammarids were fed beech leaves, Fagus sylvatica. Feeding was ceased 48-h period prior to experimentation in order to induce foraging behaviour. The three-spined sticklebacks employed during experimentation were F1 offspring of wild-caught individuals and were kept in an aerated aquarium (62 × 81 × 30 cm). In order to reduce potential confounding effects from the presence of fish faeces and residual food, the feeding (with chironomid larvae) of the three-spined sticklebacks was ceased 72 h prior to experimentation. The water in all aquaria was filtered and dechlorinated tap water (pH 7.4; EC 0.5 mS cm−1) was used.
Experimental setup
Laboratory experiments were conducted in a climate chamber at 16 ± 1°C water temperature during May, June and July in 2010. Gammarid non-host predator avoidance was assessed using a choice setup (Fig. 1) configured as a Y-shaped maze with a basal stem (5 × 5 × 15 cm) that split into two separate upper stems (5 × 5 × 10 cm). The floor of the maze was covered with 4–6 mm gravel. Tap water was circulated into the maze through the upper stems towards the main basal stem at a rate of ca.2.3 cm3 s−1. Preliminary tests with ink and salt, respectively, revealed that a flow rate of 2.3 cm3 s−1 ensured mixing of two aquaria water in the basal stem, but prevented mixing of water in the two upper stems. The inflow water originated from one of two aquaria (each 32 × 17.5 × 19 cm). One aquarium contained tap water with 10 three-spined sticklebacks introduced into the aquarium 48 h before the initiation of the experiment. The other aquarium contained tap water only. During control experiments both aquaria contained only tap water. Non-host predator avoidance was assessed in uninfected and P. minutus infected gammarids. Experiments were categorized into either ‘I’ (P. minutus infected gammarids), or ‘U’ (uninfected gammarids). Control (i.e. no three-spined sticklebacks in either aquarium) and test (i.e. one aquarium without and the other with three-spined sticklebacks) experiments were denoted ‘C’ and ‘T’, respectively. For instance, the combination UT denoted an experiment with uninfected gammarids during which one aquarium contained only tap water and the other aquarium 10 three-spined sticklebacks. Individual gammarids were randomly chosen for each trial. The number of G. pulex, G. fossarum and E. berilloni are 82, 53 and 45 (UT), 32, 36 and 26 (IT), 30, 32 and 32 (UC), 32, 31 and 32 (IC), respectively. The water of the aquaria during the test and control experiments was aerated but oxygen levels within the aquaria were not tested. The length of the gammarids was measured using imageJ (ver. 1.35, https://imagej.nih.gov/ij/index.html) from the uropod to the pedunculus along the dorsal side. The number of cystacanths of each individual gammarid was measured. Gammarids shorter than <8 mm and with more than one cystacanth were excluded as recommended by Bauer et al. (Reference Bauer, Haine, Perrot-Minnot and Rigaud2005) and Medoc and Beisel (Reference Medoc and Beisel2008) in order to avoid possible effects due to differences in size or degree of infection. Our experiment was designed to ensure ‘fresh’ fish chemical cues, which was presumed to alert the test specimens that a non-host predator was nearby.
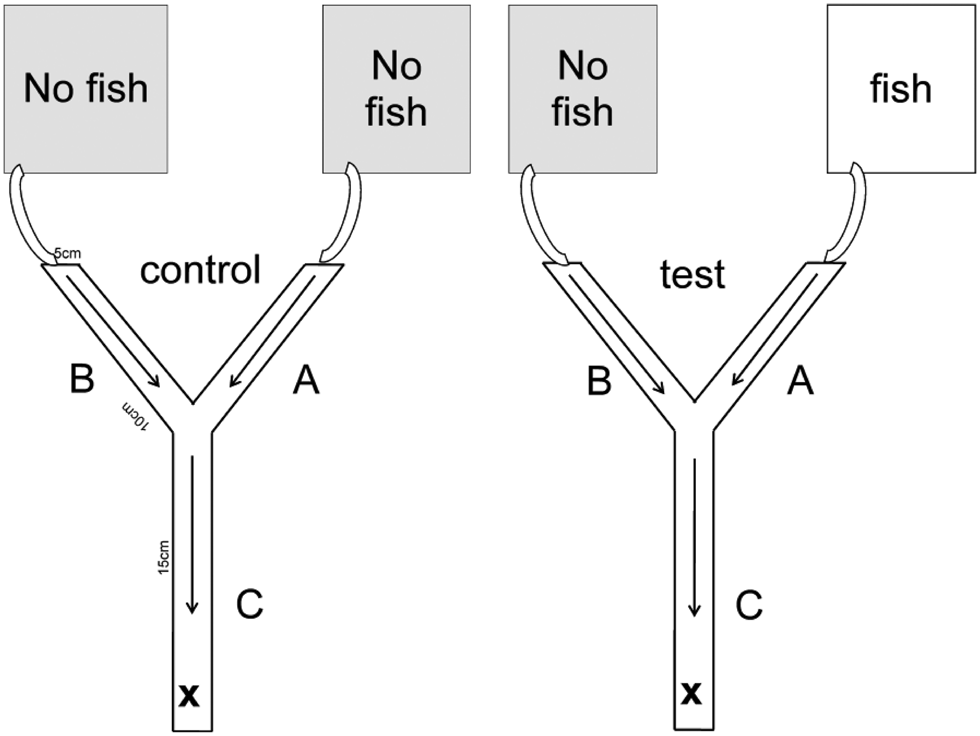
Fig. 1. Two-choice microcosm setup. A (control) = inflow from the aquarium without fish, A (test) = inflow from the aquarium with fish, B (control, test) = inflow from the aquarium without fish, C = mixing area, arrows = direction of flow, × = release point of gammarids.
Data collection
All experiments were conducted with single gammarids. The test specimen was released into the area at the base of the main stem (denoted area C, Fig. 1). Movement from area C to either areas A and B was restricted with a plastic barrier in area C for 300 s after which the test specimen could move freely within the maze for 300 s. The location of each specimen was recorded every 30th second. Specifically, the duration spent in areas C, A and B was noted (Fig. 1). The source of inflow water into areas A and B (i.e. aquaria with or without three-spined sticklebacks, respectively) was alternated between experiments. After the completion of an experiment, each specimen was re-checked for infection by other parasites. Gammarids infected with acanthocephalan parasitic worms Pomphorhynchus spp., were identified from the clearly visible orange-yellow parasites behind the transparent cuticle (Bakker et al., Reference Bakker, Mazzi and Zala1997). Infection by Echinorhynchus spp., was identifiable as an orange-/red-coloured worm visible in the pereon or the pleon (Macneil et al., Reference MacNeil, Dick, Hatcher and Dunn2003). Gammarid specimens identified as infected with parasite, other than P. minutus, or infected with P. minutus in the acanthella stage (non-infective to the definitive host) were excluded from the analysis. We did not identify other parasites in gammarids infected with P. minutus. We observed one Echinorhynchus spp. in an uninfected P. minutus individual. This individual was subsequently excluded from the analysis.
Statistical analyses
We modelled the transitions between areas A, B and C using a discrete-time Markov chain approach, i.e. an individual at location Z t = x at time t makes a transition to area Z t+1 = y at time t + 1with probability
In other words, we estimated transition matrices of the form,
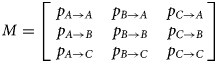
All three columns of M sum to 1; hence, M represents six independent parameters. In the full model, M was allowed to vary simultaneously with the three predictor variables: time t ∈ {1…10}, species s ∈ {EB, GF, GP} and treatment combination T ∈ {UC, IC, UT, IT}.
All specimens started from area C, and hence the first transition corresponds to two parameters (p C→A,p C→B,p C→C = 1 − p C→A − p C→B) whereas each of the subsequent nine transitions corresponded to six parameters. In total, the full model was therefore comprised of (2 + 9 × 6) × 3 × 4 = 672 parameters. Simpler models were assessed by excluding one or more predictor variables.
Given the transition probabilities p x→y, the probability Pr(Z t = y) of presence in area y at time t was estimated recursively:
Finally, given the above time-dependent probabilities, we estimated the average probability of presence at a given area as the following:
The transition probabilities in each column of M were estimated by treating the observed transition frequencies at a given area as drawn from a multinomial distribution. We fitted Bayesian Markov Chain Monte Carlo (MCMC) models with the brms interface (version 2.7.3; Bürkner, Reference Bürkner2016) to RStan (Stan Development Team, 2018), using RStudio (version 1.2.1268; RStudio Team, 2018) with R (version 3.5.0; R Development Team, 2018). We used flat priors for the transition probabilities. Each estimation was run with four MCMC chains of 2500 iterations each, preceded by a burn-in period of 1000 iterations, thus yielding a total of 10 000 samples per parameter per model. Mixing and convergence of chains was assessed from the trace plots and the Gelman−Rubin r-hat statistic (Gelman et al., Reference Gelman, Rubin, Gelman and Rubin1992). In all cases r-hat was <1.001, which suggested convergence. The effective sample size for all parameter estimates was above 1000. The mean and the 89th percentile intervals (PI) of the marginal posterior densities were reported for all parameters.
Different combinations of predictor variables were assessed using the Watanabe−Akaike Information Criterion (WAIC; Watanabe, Reference Watanabe2010). For each model m among a set of models R, we calculated the model weight according to
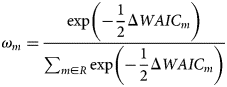
The statistic ΔWAIC m referred to the difference in WAIC value between model m and the best-fitting model (i.e. the model with the lowest WAIC score; this model itself has ΔWAIC = 0). In total, seven models each with three predictor variables were compared. The models were: species×treatment (fish presence or absence), treatment, species (amphipod species), time×treatment, time×species, time×species×treatment and time.
Results
The ‘species×treatment’ model (Table 1) was the model with the highest support at a weight of ca.1.0 among the seven competing models. No evidence of time-dependent transition probabilities was observed, i.e. all four models including time as a predictor variable were ranked lowest. In general, all test specimens tended to move away from the initial area C during the experiment and approached quasi-equilibrium frequencies in the three areas A, B and C (Fig. 2).
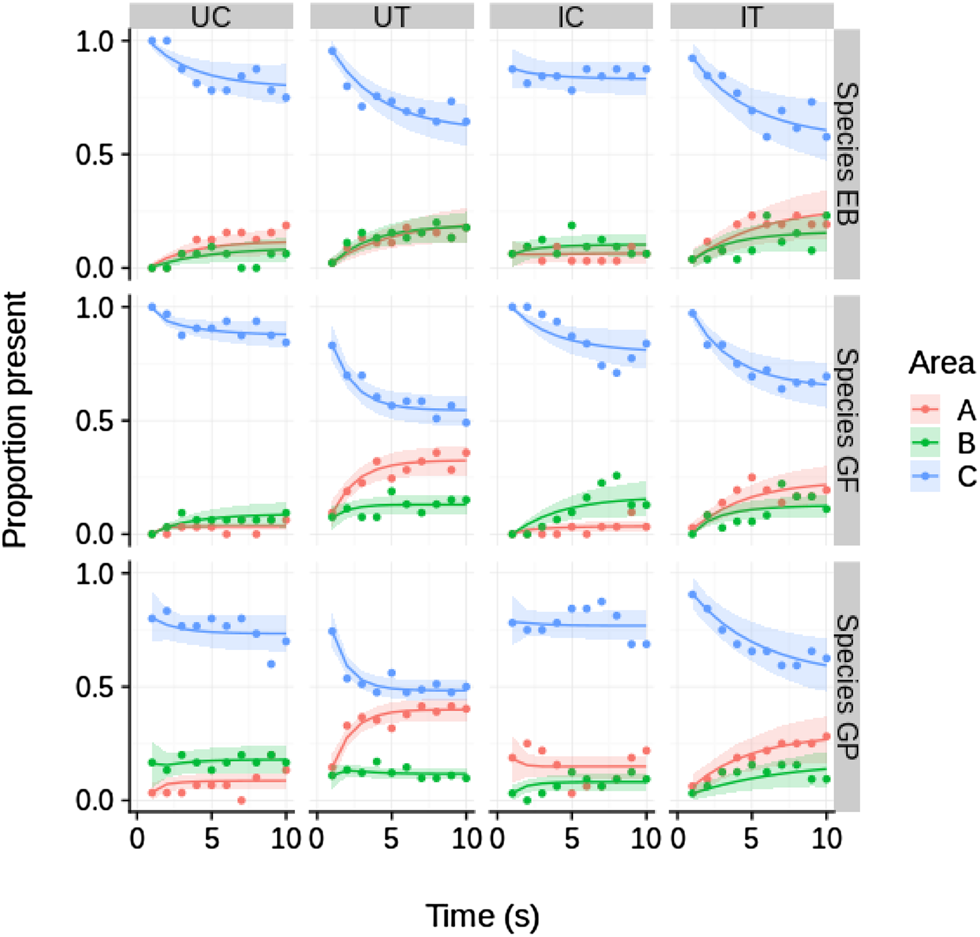
Fig. 2. Temporal dynamics of proportional presence (Y axis) in areas A, B and C of the experimental aquarium of GP (G. pulex), GF (G. fossarum), and EB (E. berilloni) for the four treatment combinations (UC, UT, IC, IT). Smoothed graphs (thick lines) are predictions of the best supported model (predictor species×treatment; see Table 1) while raw data are represented by dots. The shaded areas represent the 89% highest posterior density interval (HPDI) for the predicted values. Time (X axis) indicate 10 recordings (one per in 30 s). UC, uninfected control; UT, uninfected test; IC, infected control; IT, infected test.
Table 1. Comparison of Bayesian multinomial Markov chain models
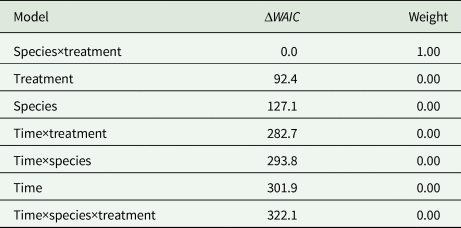
The models were ranked by their WAIC scores from low to high. ΔWAIC denotes the specific model's difference in WAIC score relatively to the model with the lowest WAIC model. Weight indicates the posterior model weight [equation (5)]
The invasive species, E. berilloni showed no statistically significant preference for area A over B or vice versa in either of the four treatment categories (Fig. 3). In contrast the native species, G. fossarum and G. pulex, showed a significant preference, mostly for area A, in six out of eight treatment categories (Fig. 3). In five out of six comparisons, between control (no non-host predator present) and test (non-host predator present) experiments, presence in area C decreased significantly when the non-host predator was present. The presence of infected G. fossarum and G. pulex in area C was more frequent in the treatment group compared to uninfected individuals (Fig. 3).
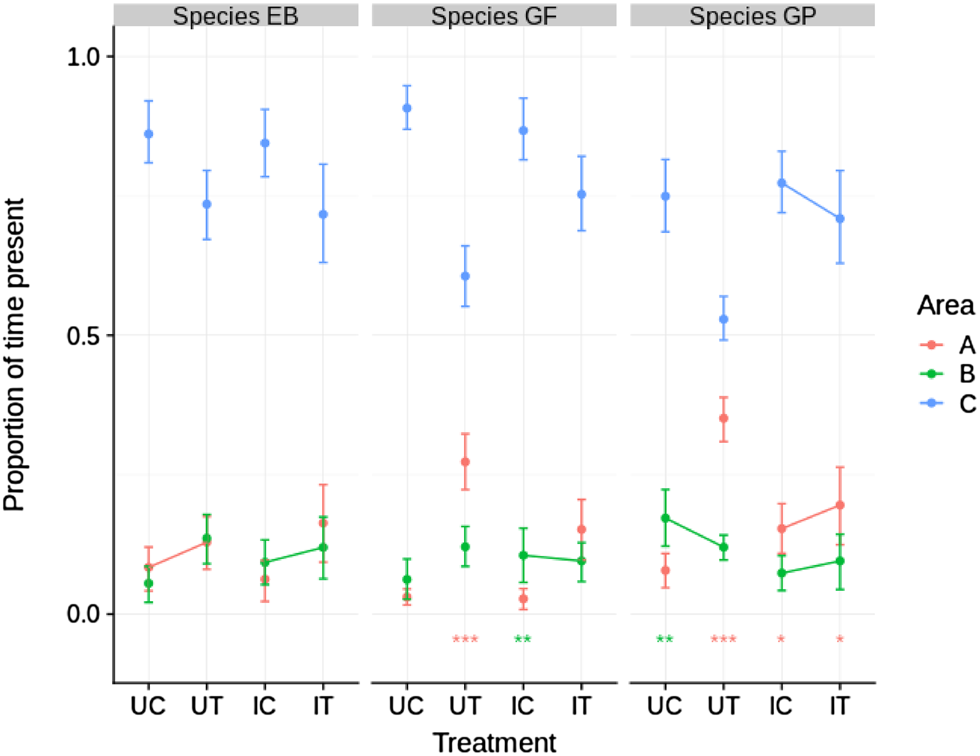
Fig. 3. Proportion of time present in different areas for all combinations of three species and four treatments. Dots indicate posterior means and error bars the 89% HPDI. Asterisks near the bottom indicate within-treatment significance of the difference between time spent at A and time spent at B, i.e. posterior probability of Pr(A) – Pr(B) >0 (red) or Pr(A) – Pr(B) <0 (green) (* P > 0.95, ** P > 0.99, *** P > 0.999). Lines between adjacent control and test treatments within areas indicate a non-significant difference (P ⩾ 0.05), whereas absence of lines indicates a significant difference (P ⩽ 0.05). UC, uninfected control; UT, uninfected test; IC, infected control; IT, infected test.
The most pronounced effect of non-host predator presence was observed in both uninfected native gammarids, with an increase in preference for area A; the area closest to the non-host predator. Infected native gammarids also had a tendency towards increased presence in area A (statistically significant for G. pulex, but not in the case of G. fossarum), although this effect was significantly lower than was the case for uninfected gammarids (posterior probabilities of Pr(A)UT – Pr(A)UC > Pr(A)IT – Pr(A)IC were 0.20, 0.99 and 1.00 for E. berilloni, G. fossarum and G. pulex, respectively).
Discussion
Avoidance behaviour of uninfected and infected gammarids to non-host predator
The estimated increase in avoidance behaviour towards the non-host predator chemical cues observed in G. fossarum infected with P. minutus in our study was consistent with the ‘increased host ability hypothesis’, which predicts that parasites increase the avoidance towards non-host predators (Medoc and Beisel, Reference Medoc and Beisel2008). In Westphalia, the native cryptic parasite P. minutus uses native G. fossarum as an intermediate host but the P. minutus co-invaded the region with invasive gammarids, E. berilloni and G. roeseli from the Mediterranean area and Southeast Europe, respectively (Zittel et al., Reference Zittel, Grabner, Wlecklik, Sures, Leese, Taraschewski and Weigand2018). Gammarus roeseli transmitted this invasive parasite to G. pulex (Zittel et al., Reference Zittel, Grabner, Wlecklik, Sures, Leese, Taraschewski and Weigand2018). In contrast, native G. pulex infected with P. minutus displayed no non-host predator avoidance perhaps because invasive P. minutus have not evolved this mechanism in the presence of the native G. pulex. Perhaps native P. minutus can potentially have different traits than invasive P. minutus in manipulating the intermediate host, G. fossarum, behaviour in such a way that it is increasing the chances of being transmitted to the definitive host. The weak effects on non-host predator avoidance in infected gammarids detected in our study could be due to the fact that our experiments were conducted without the host predator chemical cues, making it more difficult for individuals to discern between different predator chemical cues and to behave accordingly. Our results would probably be different if we included both host predator and non-host predator in our experiment, e.g. Jacquin et al., Reference Jacquin, Mori, Pause, Steffen and Medoc2014. We agree that it is worthwhile to carefully contemplate when it is appropriate to make the inference regarding the specificity of gammarid behaviour in relation to ‘non-host’ predators vs predators in general. Since our experiments did not include a host predator we were unable to compare behavioural responses towards these two predator groups. Accordingly, we cannot state with any certainty, that the observed responses were specifically due to non-host predator presence, i.e. the behaviour could have been elicited because of predator presence per se.
The realization of the presence of the predator by the prey possibly occurs indirectly through the detection of pheromones and chemical cues from other prey species released during predation (Dicke and Grostal, Reference Dicke and Grostal2001) or directly via chemical cues from the predator (Kats and Dill, Reference Kats and Dill1998). In aquatic environments, the prey detects predators mostly via chemical cues (Dodson et al., Reference Dodson, Crowl, Peckarsky, Kats, Covich and Culp1994; Chivers et al., Reference Chivers, Brown and Smith1996; Hettyey et al., Reference Hettyey, Zsarnóczai, Vincze, Hoi and Laurila2010). For example, gammarids respond to chemical cues from predators at low visibility by reducing activity and move towards the bottom (Wisenden et al., Reference Wisenden, Cline and Sparkes1999).
This study also revealed that uninfected gammarids had a preference towards non-host predators. This behaviour was especially noticeable in the native gammarids compared to the invasive gammarid. However, these results did not agree fully with our expectations, as native gammarids preferentially moved towards non-host predator chemical cues whereas invasive gammarids reduced their activity in the presence of non-host predator chemical cues but did not appear to actively avoid non-host predator chemical cues. The results of this study were in agreement with those previously reported by Schmidt-Drewello et al. (Reference Schmidt-Drewello, Riss, Scharsack and Meyer2016), who found that uninfected invasive E. berilloni appeared more capable of detecting chemical cues from three-spined stickleback compared to uninfected native gammarids, G. pulex and G. fossarum. There may be several causes for the observed absence of predator avoidance towards non-host predators by uninfected native gammarids. Uninfected native gammarids appear able to discern between the chemical cues emitted by efficient predators [specialist predators with using efficient hunting behaviours and persisting to eat preferred prey items (Spencer et al., Reference Spencer, Newsome and Dickman2017) e.g. sculpin, Cottus gobio, trout, Salmo trutta, in the Stampen Stream in southern Sweden (Abjornsson et al., Reference Abjornsson, Dahl, Nyström and Brönmark2000), perch, Perca fluviatilis, Lake Lucerne in Switzerland (Baldauf et al., Reference Baldauf, Thünken, Frommen, Bakker, Heupel and Kullmann2007) and gudgeon, Gobio gobio, in Gauernitzbach near Dresden,Germany (Szokoli et al., Reference Szokoli, Winkelmann, Berendonk and Worischka2015)] and those chemical cues emitted by inefficient predators, i.e. scavengers or predatory species that are inefficient in targeting gammarids [e.g. cray fish, Pacifastacus leniusculus, in the Stampen Stream (Abjornsson et al., Reference Abjornsson, Dahl, Nyström and Brönmark2000), stone loach, Barbatula barbatula, in Gauernitzbach (Szokoli et al., Reference Szokoli, Winkelmann, Berendonk and Worischka2015) and three-spined sticklebacks in Lunain river at Nonville, France (Jacquin et al., Reference Jacquin, Mori, Pause, Steffen and Medoc2014)]. Our results may suggest the underlying cause of the observation that uninfected, invasive gammarids limit their range to areas with fish predators relative to the range of uninfected native gammarids on the Paderborn Plateau (Keane and Crawley, Reference Keane and Crawley2002). However, this needs further investigation. Another cause for the observed absence of predator avoidance towards non-host predators by uninfected native G. pulex is its local adaptation in the presence of fish.
Gammarus pulex is an active and explorative amphipod (Truhlar and Aldridge, Reference Truhlar and Aldridge2015). It lives in shallow muddy streams where low water depth and dense benthic detritus may reduce the risk of detection by fish (Haddaway et al., Reference Haddaway, Vieille, Mortimer, Christmas and Dunn2014). Gammarus fossarum is abundant in the upper reaches of streams or in the mountainous areas, where the only predatory fishes are salmonids. Presumably, G. fossarum has not been exposed to a diverse array of fish predators (Siegismund and Müller, Reference Siegismund and Müller1991; Müller, Reference Müller2000; Pöckl et al., Reference Pöckl, Building, Drive, House and Sawrey2003).
Fish chemical cues could signal the presence of potential food resources to gammarids, Gammarids are omnivore scavengers that feed on carcasses and fish faeces (Wilhelm and Schindler, Reference Wilhelm and Schindler2011; Jermacz et al., Reference Jermacz, Dzierżyńska-Białończyk and Kobak2017). However, our results were not confounded by fish chemical cues. The three-spined sticklebacks in our experiment, were not fed 72 h prior to the experiments in order to remove such chemical cues (Wisenden et al., Reference Wisenden, Cline and Sparkes1999; Ward, Reference Ward2012; Smith and Webster, Reference Smith and Webster2015). In our experiment, the presence of fish faeces was not recorded. However, given the potential significance of fish faeces as food for amphipods, future studies should attempt to disentangle whether fish chemical cues and/or the presence of fish faecal matter influences gammarid behaviour.
Our experiment employed chemical cues from non-host predator that were present in the vicinity of the gammarid during the experiment, presumably alerting the individual gammarid of their proximity. Indeed, the presence of infected and uninfected gammarids in the release area (C) decreased (but not always) when ‘fresh’ chemical cues of non-host predator were present. Unwillingness to presence in the release area was a specific case and confirmed increasing gammarids activity after detecting the source of chemical cues and confirmed increasing the time duration of presence of infected and uninfected gammarids in area with non-host predator chemical cues. Our results were in contrast with previous studies that demonstrated a decrease in prey activity when predators were present, such as, reduction feeding activity [e.g. in tadpoles, Rana temporaria (Van Buskirk et al., Reference Van Buskirk, Krügel, Kunz, Miss and Stamm2014)] and swimming, crawling and downstream drift [e.g. in Hyalella azteca (Stone and Moore, Reference Stone and Moore2014), Gammarus lacustris (Wudkevich et al., Reference Wudkevich, Wisenden, Chivers and Smith1997) and Echinogammarus stammeri (Dezfuli et al., Reference Dezfuli, Maynard and Wellnitz2003)].
Intra-specific comparison between uninfected and infected gammarids
No preference for areas with and without three-spined stickleback chemical cues was observed in either uninfected or infected G. pulex. This observation was in agreement with previous reports by Kaldonski et al. (Reference Kaldonski, Perrot-Minnot and Cézilly2007), who did not detect any significant differences in the use of refuges between infected or uninfected G. pulex to chemical cues from freshwater sculpin, Cottus gobio, a non-host predator. However, Kaldonski et al. (Reference Kaldonski, Perrot-Minnot, Motreuil and Cézilly2008), showed that water scorpions, Nepa cinera, predated upon uninfected G. pulex more frequently than infected G. pulex.
We found that infection by P. minutus altered the non-host predator avoidance in native G. fossarum, in a manner that decreased the probability of presence in the area with three-spined stickleback chemical cues, compared to uninfected G. fossarum. Conversely, neither infected nor uninfected E. berilloni showed a significant preference for a specific area (with or without three-spined stickleback chemical cues) in either of the four treatment categories. Jacquin et al. (Reference Jacquin, Mori, Pause, Steffen and Medoc2014) found that infected E. berilloni by P. minutus under the adaptationist hypothesis (Baldauf et al., Reference Baldauf, Thünken, Frommen, Bakker, Heupel and Kullmann2007) reduced their activity in the presence of fish chemical cues, although modulating the intermediate host's behaviour is not always adaptive for the parasite (Bakker et al., Reference Bakker, Frommen and Thünken2017). However, Jacquin et al. (Reference Jacquin, Mori, Pause, Steffen and Medoc2014) employed native E. berilloni from the Lunain river (France) and non-host predator chemical cues from both three-spined stickleback and minnows, Phoxinus phoxinus. Our findings indicated that there was a difference in non-host predator avoidance behaviour of infected E. berilloni on the Paderborn Plateau in comparison to their native origin area in France.
The outcome of interactions between native and invasive hosts and their parasites are shaped by an array of complex ecological and evolutionary factors (Alexander et al., Reference Alexander, Thrall, Antonovics, Jarosz and Oudemans1996; Parker and Gilbert, Reference Parker and Gilbert2004) affecting the success and intensity of invasions by non-native species (MacNeil et al., Reference MacNeil, Dick, Hatcher and Dunn2003; Prenter et al., Reference Prenter, MacNeil, Dick and Dunn2004). The fate of invasive species is, in part, governed by competition between native and invasive species according to the ‘evolution of increased competitive ability’ hypothesis (Blossey and Nötzold, Reference Blossey and Nötzold1995) as well as competition between uninfected and infected hosts during the invasion according to the ‘increased host ability hypothesis’ in order to reach the parasite maximum fitness and enhance their transmission to the next host e.g. strong anti-predator response and highest escape speed in infected G. roeseli by P. minutus towards non-host crustacean predator Dikerogammarus villosus in order prevent inappropriate transmission (Medoc and Beisel, Reference Medoc and Beisel2008; Beisel and Médoc, Reference Beisel and Médoc2010). Natural selection is assumed to act on traits that enables the parasite to manipulate its intermediate host's phenotype in a manner that increases the probability of trophic transmission of the parasite to the definitive host (Thomas et al., Reference Thomas, Adamo and Moore2005). One possible mechanism is manipulation of the intermediate hosts avoidance behaviour towards non-host predators in order to reduce the chance of the intermediate host by predators that are not definitive hosts, presumably increasing the likelihood of predation by the definitive host. The ultimate cause of ‘increased parasite fitness through transmission to a definitive host-predator’ occurs in combination with proximate causes of the success or failure of infected intermediate hosts, such as avoidance of non-host predators.
During the test and control experiments the level of oxygen in aquaria was not monitored. The treatment with the fish cues would likely have had lower levels of oxygen. It could affect the gammarids decision to whether select areas with fish chemical cues that contains low dissolved oxygen or without fish chemical cues that includes high level of dissolved oxygen.
Conclusion and recommendation
Our study revealed that parasites are capable of increasing non-host predator avoidance in G. fossarum. However, no effects in non-host predator avoidance was observed in uninfected or infected G. pulex and E. berilloni. Other studies have demonstrated modulation of host behaviour by P. minutus specifically in sympatric gammarids. For example, a more pronounced change in geotaxis was shown in native G. pulex infected by P. minutus compared to an infected invasive host, G. roeselii (Bauer et al., Reference Bauer, Haine, Perrot-Minnot and Rigaud2005). P. minutus appears able to exploit the plasticity of sympatric intermediate host antipredator responses to its own advantage. Accordingly, care should be taken to ensure that species of host and species of parasite are not confounded, especially since one intermediate host species (G. pulex) was sampled from another river than the remaining intermediate host species (G. fossarum and E. berilloni).
Zittel et al. (Reference Zittel, Grabner, Wlecklik, Sures, Leese, Taraschewski and Weigand2018) showed that the three gammarids in this study are intermediate host to a cryptic P. minutus ‘species’. Accordingly, Zittel et al. (Reference Zittel, Grabner, Wlecklik, Sures, Leese, Taraschewski and Weigand2018) recommended genetic identification of P. minutus, in order to ascertain if differences in observations may be attributed to different P. minutus species. Indeed, intermediate host species with different P. minutus species have different non-host predator avoidance behaviour. In other words, invasive intermediate hosts may introduce invasive parasites into the native intermediate host population possibly resulting in native parasite maladaptation in invasive new host species (Moret et al., Reference Moret, Bollache, Wattier and Rigaud2007). However, it is questionable if the ‘increased host ability hypothesis’ would vary on the cryptic species of the parasite. Water fowl is a vector for P. minutus between native and invasive gammarids in the Paderborn Plateau. The lower susceptibility to non-final host predators of infected G. fossarum could facilitate invasion by G. fossarum into the upper parts of the streams, and hence aid in the establishment of G. fossarum and non-native P. minutus in the ecological niche currently occupied by native gammarids by competitive exclusion. Our results may have implications in terms of the role of parasites in the success or failure of invasive species to colonize new areas.
Recent studies showed that behavioural plasticity (individual differences in behaviour) and consistency (consistent among-individual variation in behaviour) of intermediate hosts differed among both host sex and infection status (Park and Sparkes, Reference Park and Sparkes2017). Unfortunately, we were unable to test sex-specific behaviour of amphipods in our study, because we did not have the time to identify the sexes of individuals. We conclude that uninfected gammarids did not appear to actively avoid non-host predators. We recommend to conduct experiments with different non-host predators and host predators in order to assess differences in predators' efficiency and coevolution history of the prey−predator.
Acknowledgements
We are grateful to Frank Groenewoud, Sjouke Anne Kingma and Martijn Hammers for their constructive comments on the paper. We acknowledge the Institute for Evolution and Biodiversity, University of Münster, Germany, for providing permission and the facilities to conduct the research study, and students for their assistance during field sampling.
Financial support
This work was supported by the Netherlands Organisation for Scientific Research grant numbers 854.11.003 and 823.01.014 (JK).
Data availability statement
The R-code and Stan-code is available at GitHub (https://github.com/idopen/Gammarid_predator_avoidance).
Conflict of interest
None.
Ethical standards
Not applicable.







