Introduction
The effects on the reproduction capacity of bivalves under pathological infections have been extensively studied since it is a major cause of production and recruitment decrease in natural marine and estuarine beds (Dittman et al., Reference Dittman, Ford and Padilla2001; Villalba et al., Reference Villalba, Reece, Ordás, Casas and Figueras2004). Parasites’ impacts can vary from deleterious effects on animal reproduction to complete neutering, as it is usually the case in bivalves infected by trematodes (Kabat, Reference Kabat1986; Brian and Aldridge, Reference Brian and Aldridge2020; Magalhães et al., Reference Magalhães, Freitas and de Montaudouin2020). Other parasites have a lower effect on reproduction, but they can also greatly impact the host's performance. In the case of Haplosporidian parasites such as Haplosporidium nelsoni, a delay in gametogenesis development of the host and also a lower egg production were described (Ford and Figueras, Reference Ford and Figueras1988), while the effect of other parasites of the same group, such as Bonamia ostreae and B. exitiosa, on gametogenesis is not clear (da Silva et al., Reference da Silva, Fuentes and Villalba2009; Heenan, Reference Heenan2020). Intrahaemocytic parasites such as Perkinsus marinus can cause a delay in the gonad development of the oyster Crassostrea virginica but reproductive capacity and spawning seem to be unaffected (Kennedy et al., Reference Kennedy, Newell, Krantz and Otto1995; Dittman et al., Reference Dittman, Ford and Padilla2001).
Perkinsus olseni is an alveolate parasite that causes deleterious effects on several species of bivalves. First described in the gastropod Haliotis rubra (Lester and Davis, Reference Lester and Davis1981), it is now a major concern for clam production worldwide (Villalba et al., Reference Villalba, Reece, Ordás, Casas and Figueras2004; Choi and Park, Reference Choi, Park, Ishimatsu and Lie2010). This parasite can provoke severe effects on the host's physiology, leading to an energetic imbalance, a delay in gonad maturation and impacts on reproduction that could lead to massive mortality or infection by other pathogens due to a reduction in the immune defence capacity (Park et al., Reference Park, Figueras and Choi2006; Villalba et al., Reference Villalba, Gestal, Casas and Figueras2011; Casas and Villalba, Reference Casas and Villalba2012; Soudant et al., Reference Soudant and Volety2013). Mass mortality in Ruditapes decussatus clams associated with P. olseni infection was reported in several countries: Spain with up to 100% mortality in Delta de L´Ebre (Santmartí et al., Reference Santmartí, Montes, Pech, Durfort, Castelló and Calderer1995), Portugal with 50–85% mortality observed in Ria Formosa (Ruano et al., Reference Ruano, Batista and Arcangeli2015) and Italy with P. olseni prevalent in moribund individuals (Ramilo et al., Reference Ramilo, Carrasco, Reece, Valencia, Grau, Aceituno, Rojas, Gairin, Furones, Abollo and Villalba2015). Mortality impacting Ruditapes philippinarum has also been reported in Italy and Korea (Choi and Park, Reference Choi, Park, Ishimatsu and Lie2010; Pretto et al., Reference Pretto, Zambon, Civettini, Caburlotto, Boffo, Rossetti and Arcangeli2014), illustrating the magnitude of the problem for clam production worldwide.
The effects of Perkinsus spp. on host physiology were largely evaluated. In C. virginica oysters infected with P. marinus, it was reported that a large number of parasites can consume more energy than the oyster is able to produce, leading to a negative energy balance of the host (Choi et al., Reference Choi, Wilson, Lewis, Powell and Ray1989). The same effect was measured in R. decussatus infected with P. olseni but with no clear effect on spawning efficiency or fecundity (Casas, Reference Casas2002; Villalba et al., Reference Villalba, Gestal, Casas and Figueras2011; Casas and Villalba, Reference Casas and Villalba2012). This energetic imbalance resulted in the affected individuals’ slower growth and a lower condition index (CI) (Burreson and Ragone Calvo, Reference Burreson and Ragone Calvo1996; Leite et al., Reference Leite, Afonso and Cancela2004; Casas and Villalba, Reference Casas and Villalba2012). If the infection invades the gonad and the surrounding follicles, occupying their space, the reproductive capacity of the host can also be compromised. In R. philippinarum infected with P. olseni, egg production was shown to be reduced in heavily infected clams, resulting in poorer spawning efficiency and clam recruitment (Park et al., Reference Park, Figueras and Choi2006).
The present study aimed to evaluate the effect of P. olseni on the reproduction of R. decussatus, a highly important commercial clam species from the Mediterranean and Northeast Atlantic regions. For that, naïve clams were injected with two doses of parasites, low and high infection under laboratory conditions. Their maturation, CI, biochemical performance, spawning capacity and larvae viability were evaluated during a 2-month trial. This research is included in a broader project which aims to develop a robust protocol for seed yield and increase the production capacity of native species.
Materials and methods
Clam collection and P. olseni injection and diagnosis
Around 720 adult R. decussatus clams (43.95 ± 2.89 mm) were collected in March 2021 from a P. olseni-free natural bed in Testal beach, Noia (42.791184, −8.914513; Spain). They were transported at 15°C in freezer bags to Oceano Fresco S.A. facilities [Nazaré (39.581626, −9.076963), Portugal] and conditioning for 3 weeks (18 ± 0.5°C, 35 ppm) before the start of the experiment. The parasite-free status was further verified on 15 clams using the Ray's Fluid Thioglicolate Method (RFTM) method (Ray, Reference Ray1952; Villalba et al., Reference Villalba, Casas, López and Carballal2005), which stablished the infection scale: level 0 – absence of parasite, 1 – very slight infection, 2 – slight infection, 3 – moderate infection, 4 – intensive infection, 5 – very intensive infection. For the experimental challenge, clams were notched in the shell next to the anterior adductor muscle (Fig. 1). Then, clams were randomly divided into 3 groups and injected with 100 μL of P. olseni 5×104 suspension (5×103 cells per clam) for the low infection (LI) group, and 100 μL of P. olseni 5×106 suspension (5×105 cells per clam) for high infection (HI) group. Controls (CTL) were injected with 100 μL of filtered sea water (FSW) according to the protocol described in Garcia et al. (Reference Garcia, Estêvão, Costas, Cruz and Fernández-Boo2022).
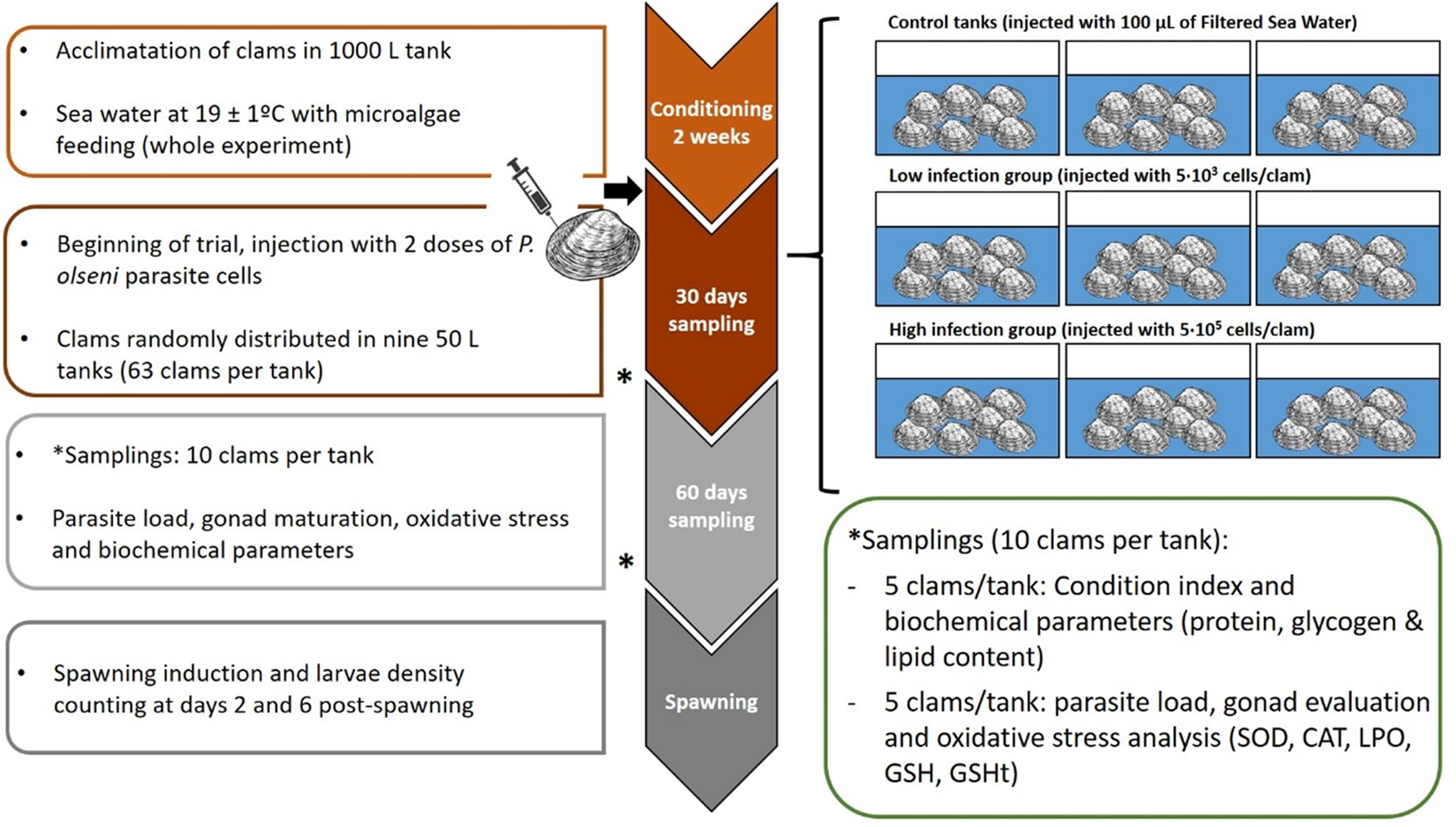
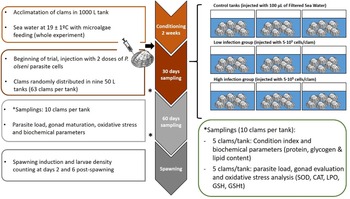
Fig. 1. Experimental design of the experience. Three groups were evaluated (control, low and high infected) and two samplings were done during experience at days 30 and 60 post-injection. Spawning was induced after 60 days; fecundity and larval viability were evaluated.
After injection, clams were transferred to 50 L tanks (63 clams per tank), 3 tanks per condition (CTL, LI, HI), with aerated 1 μm FSW, at 19 ± 1°C with a mixed feeding of Diacronema lutheri, Isochrysis galbana clone T-iso, Chaetoceros gracilis and Tetraselmis chuii, in a proportion of 1:1:1:1 based on size terms. This conditioning is commonly used in hatcheries to create the optimal conditions for bivalve gonad maturation and to obtain larvae out of the natural spawning season. A total of 30 clams per condition (10 clams per tank) were sampled on days 0, 30 and 60 after challenge (Fig. 1). Fifteen clams per condition (5 clams per tank) were frozen directly at −20°C for CI and gross biochemical parameters evaluation such as protein, glycogen and lipid content. The remaining 15 sampled clams (5 clams per tank) were used for histological evaluation of gonadal development, oxidative stress parameters evaluation and diagnosis of P. olseni infection intensity by RFTM method using one hemi-gill according to Villalba et al. (Reference Villalba, Casas, López and Carballal2005). Mortality was recorded during the whole experiment.
Condition index and whole-body composition
For condition index (CI) determination, the dry meat and shell weight of 15 clams per condition (5 clams per tank) from each sampling period were determined after oven drying at 80°C for 24 h. Meat samples were then turned into ash at 450°C for 24 h in a muffle furnace, ash weight determined and organic matter weight calculated as the ash-free dry meat weight (AFDW). The CI was calculated according to Walne and Mann (Reference Walne, Mann and Barnes1975): [AFDW (g)/dry shell weight (g)] × 100.
For gross biochemical composition determination, briefly, for each specimen, the protein content was determined using the modified Lowry method (Shakir et al., Reference Shakir, Audilet, Drake and Shakir1994), glycogen content was determined from dried homogenate (80°C for 24 h) using the anthrone reagent (Viles and Silverman, Reference Viles and Silverman1949) and total lipids were extracted from fresh homogenized material in chloroform/methanol (Folch et al., Reference Folch, Lees and Sloane Stanley1957) and estimated spectrophotometrically after charring with concentrated sulphuric acid (Marsh and Weinstein, Reference Marsh and Weinstein1966). Duplicate determinations were performed in all cases and values were expressed as a percentage of AFDW.
Histological analysis
Fifteen individuals per condition from each sampling period were examined histologically to determine the gametogenic stage. A small section of the visceral mass was separated from siphons and gills and fixed in Davison solution for 48 h, then transferred to 70% ethyl alcohol for storage. Tissues from these samples were dehydrated with serial dilutions of alcohol and embedded in paraffin. Sections (6–8 μm) were cut on a microtome and stained with haematoxylin and eosin. The histologically prepared slides were examined and each specimen was assigned to a sex and gonadal stage according to Delgado and Pérez-Camacho (Reference Delgado and Pérez-Camacho2007). When more than one developmental stage occurred simultaneously within a single individual, the assignment of a stage criteria decision was based upon the condition of the majority of the section according to Matias et al. (Reference Matias, Joaquim, Matias, Moura, de Sousa, Sobral and Leitão2013).
Oxidative stress parameters determination
After histological sampling, remaining soft tissues were manually homogenized with liquid nitrogen, using a mortar and a pestle, and separated into subsamples (3 subsamples with 0.5 g) for further analysis. For each parameter, extraction was performed with a specific buffer in a 1:2 (w/v) ratio with the previously homogenized tissue as described in Magalhães et al. (Reference Magalhães, de Montaudouin, Figueira and Freitas2018). Protein (PROT) content, catalase (CAT) and superoxide dismutase (SOD) activities, lipid peroxidation (LPO) level, Electron transport system (ETS), total glutathione (GSHt) and reduced glutathione (GSH) contents were determined according to Magalhães et al. (Reference Magalhães, de Montaudouin, Figueira and Freitas2018).
Spawning and larval viability
After 60 days of the experiment, 73 clams from the CTL, 74 from LI and 65 from HI groups were induced to spawn by thermal stimulation, through five cycles of a rapid increase of temperature from 15 to 25 ± 1°C over a 1 h interval, recovery for 30 min at 15 ± 1°C and repeat the increasing temperature following the protocol previously established by Matias et al. (Reference Matias, Joaquim, Leitão and Massapina2008). Embryos from each treatment were incubated in 450 L tanks, with 1 μm-filtered seawater, maintained at 19 ± 0.5°C, at a density of around 20 embryos per mL. After 48 h, the number of D-larvae was determined. D-larvae were then reared in 450 L tanks. During larval rearing (around 10 larvae per mL), water was renewed 3 times a week (19 ± 0.5°C). Before the water change (days 2, 4 and 6 post-spawning), to estimate the survival, larvae were counted in 5 10 mL samples. After each water renewal change, all treatments received an equal volume of phytoplankton cells. The larval diet constituted 40 cells per μL of a microalgae mix composed of D. lutheri, I. galbana clone T-iso, C. gracilis and T. chuii, in equal proportion based on size terms.
Statistical treatment of data
All statistical analyses were performed using SPSSv.26 statistical analysis software (IBM, USA). Cumulative mortality of progenitors was evaluated using a Kaplan–Meier curve with a log-rank test for group comparisons.
Results were expressed as mean ± standard deviation (s.d.). After verifying normality and variance homogeneity, a one-way analysis of variance was carried out. Tukey's Honest Significant Difference (HSD) post hoc test was performed to identify significant differences among groups with a level of significance of P < 0.05.
Results
Infection intensity and mortality
The evaluation of the infection level of P. olseni by the RFTM technique showed no infection in control groups at any sampling point, while, at day 60, an average of 2.4 ± 0.9 and a 4.86 ± 0.35 level were observed in LI and HI groups, respectively. No significant differences were observed between samples of the same group between days 30 and 60 post-injection, but significant differences were detected between LI and HI groups (P < 0.001) (Fig. 2).
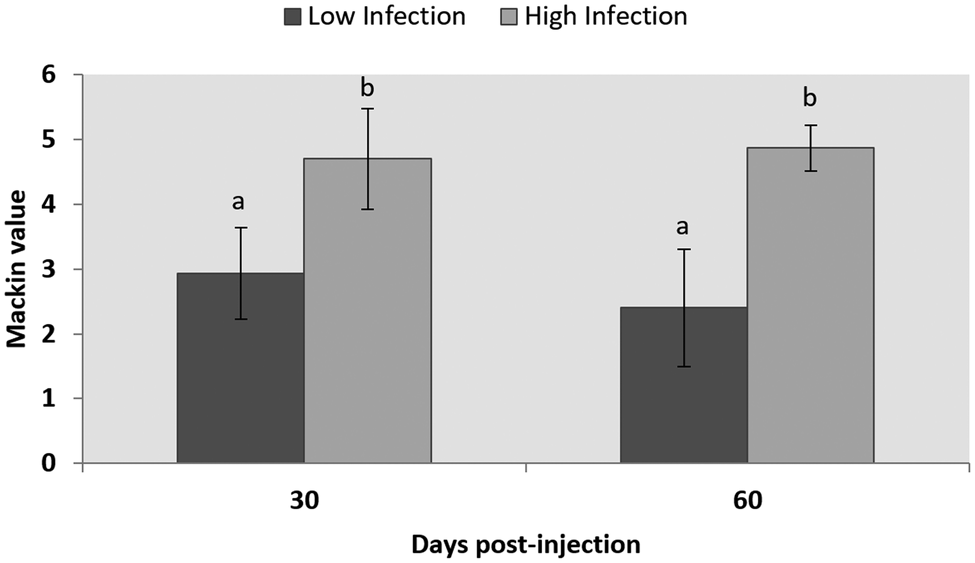
Fig. 2. Infection level according to the Mackin scale of LI and HI groups after 30- and 60 days post-injection. Results are shown as mean ± s.d. Letters show statistically significant differences among groups (P < 0.05).
During the 60 days of the experiment, mortality occurred under all treatments, especially during the first 30 days of maturation. The control group presented a cumulative mortality of 9.5% while the LI group reached 21.2% and the HI group 16.2% (Fig. 3). Statistically significant differences were found between the infected groups and the control group (LI vs CTL, P = 0.020; HI vs CTL, P = 0.018), but no significant differences were found between low and high infected groups (P = 0.431).
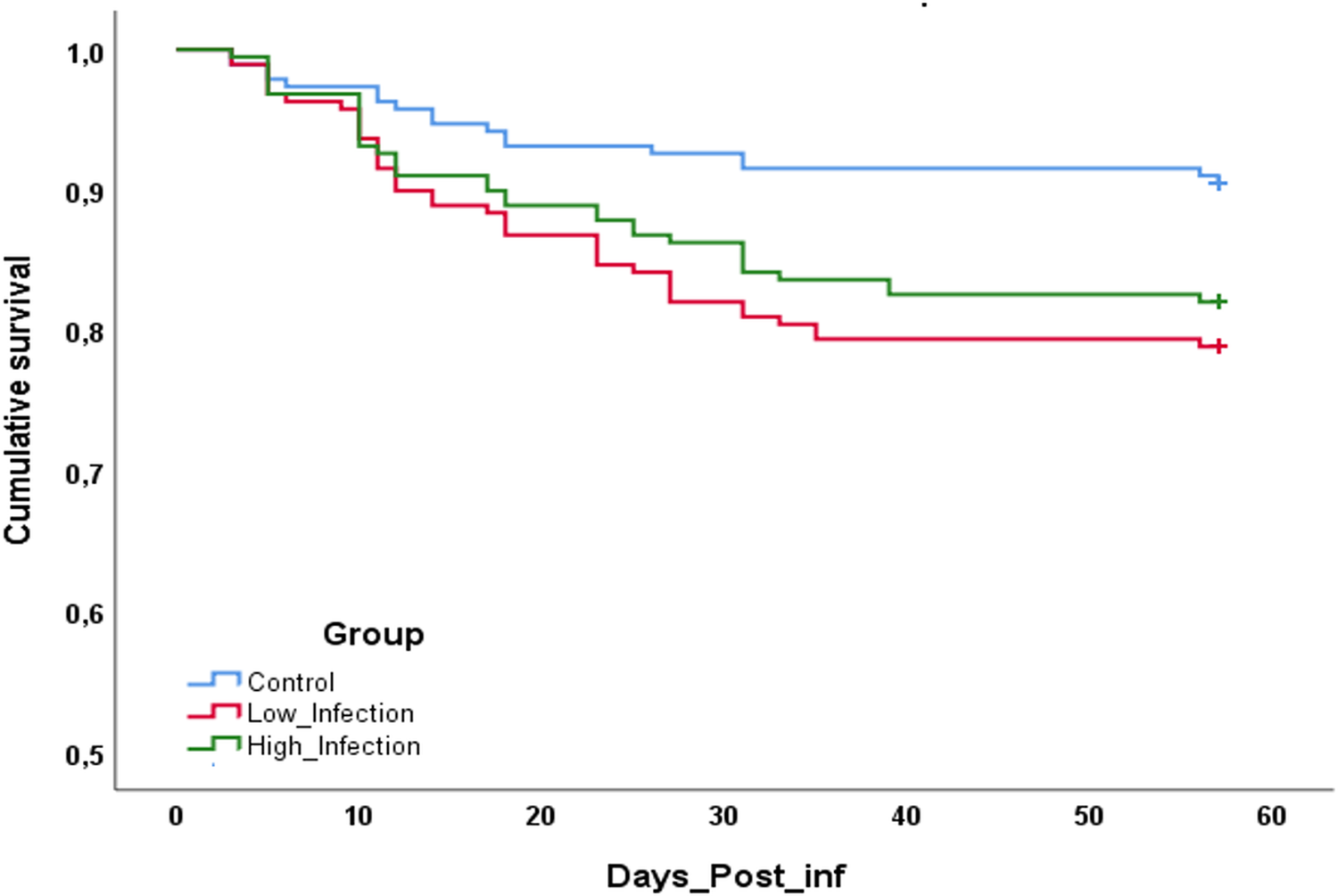
Fig. 3. Cumulative survival of clams after 60 days of experience. *Indicates statistically significant differences against control condition (P < 0.05).
Condition index and whole-body gross biochemical composition
CI showed significant differences among groups and the sampling period. The maximum CI was found in control clams at time 0, before conditioning, with a value of 11.28. However, these values decreased significantly in the first month with a minimum CI value measured at LI_T1 group with 8.31 (Table 1), which was slightly lower than clams from control and high infected group at the same time, but without significant differences. At the end of the experiment, CI varied from 8.73 in the control group to 9.68 in the HI group, however there were no significant differences among treatments.
Table 1. Values of condition index, protein, glycogen and lipid during conditioning
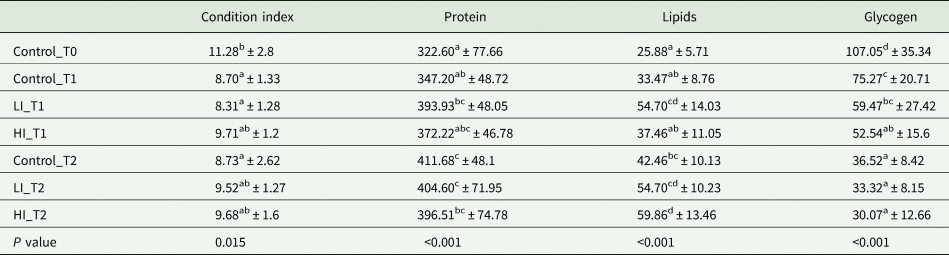
Values of gross biochemical composition are expressed as μg mg−1 of ash-free dry meat weight (AFDW). Data are expressed in mean ± s.d. Superscript letters represent significant differences among groups (P < 0.05).
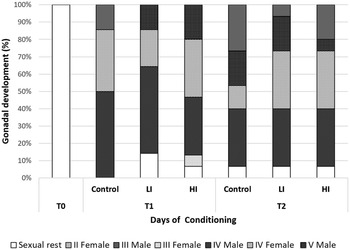
Regarding histology, at T0 the gonads from all clams were on sexual rest. After 30 days of conditioning, most of the clams were already in full maturation in all treatments; however, some differences were found between the control and infected groups (Fig. 4). The control group was the most advanced one in sexual maturation with 85.71%, while 14 and 29% of the individuals were in stages IV (ripe) and V (partially spawned) of gonad development, respectively. Individual reaching stage V was only observed in the control group at 30 days post-infection. In low and high infected groups, the percentage of stage IV was 71.43 and 66.66%, respectively. Also, some clams were still in sexual rest in infected groups at 30 days post-infection (LI = 14.29%; HI = 6.67%). HI clams showed higher variability, what appears to be a delay in gonadal development. At the end of conditioning, 60 days post-infection, the proportion of sexually mature individuals decreased in control and LI clams, and partially spawning (stage V) increased in all groups (46.67% in the control and 26.67% in both infected groups). Also, a small percentage of 6.67% of clams in sexual rest still appeared in all treatments.
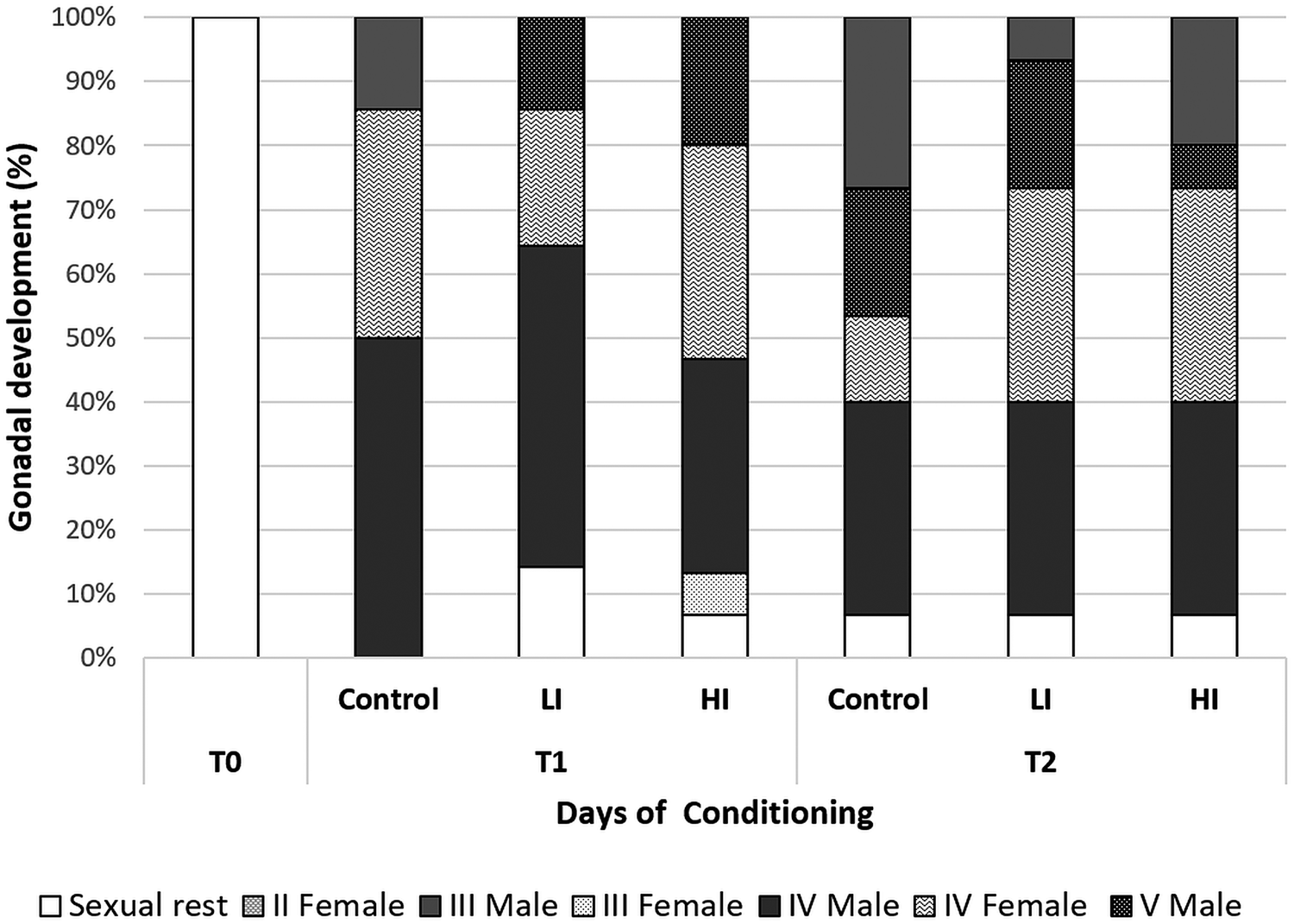
Fig. 4. Gonad development stage of clams at each condition and sampling time. T1: 30 days post-infection, T2: 60 days post-infection.
Regarding gross biochemical composition, protein, total lipid and glycogen contents showed differences among groups (Table 1; Fig. 5). Energy reserves, in glycogen form, were higher at the beginning of the experiment (107.05 ± 35.34 μg mg−1 AFDW). After 30 days, the infected clams showed lower glycogen content (59.47 ± 27.42 and 52.55 ± 15.60 μg mg−1 AFDW, for LI_T1 and HI_T1, respectively) compared with the individuals under control conditions (75.27 ± 20.71 μg mg−1 AFDW) but differences were only significant between control and HI groups. At the end of the experiment, no significant differences were observed among conditions. The lowest protein and lipid content were present in control clams at time 0 in which all clams were in the sexual rest stage of gonad development. The maximum values were recorded in LI_T2 and HI_T2, when glycogen minimum values were found (36.52 ± 8.42, 33.32 ± 8.15 and 30.08 ± 12.66 μg mg−1 AFDW, in C_T2, LI_T2 and HI_T2, respectively).
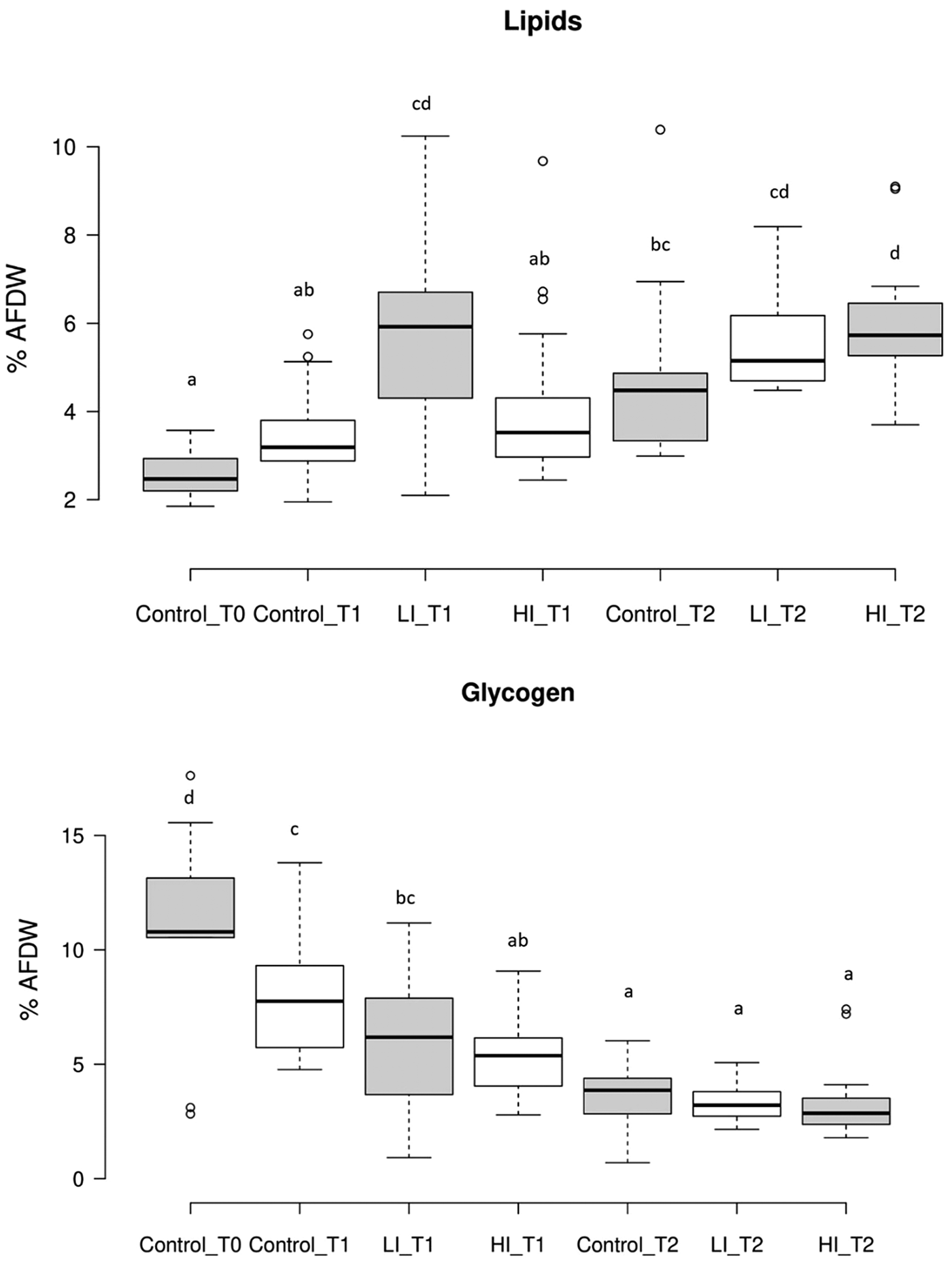
Fig. 5. Results of lipid and glycogen content present as % of ash-free dry weight (AFDW) of meat (g)/dry shell weight (g). Letters show statistically significant differences among groups (P < 0.05). T1: 30 days post-infection, T2: 60 days post-infection.
Oxidative stress parameters
No differences were found in the ETS activity and reduced GSH content, regardless of the treatment and the sampling period tested. On the other hand, differences were found in LPO levels during the experimental period with a maximum value in Control_T0 (203.36 ± 43.02 nmol MDA g−1) and a minimum in HI_T2 (81.68 ± 22.90 nmol MDA g−1); while for a given sampling period, significant differences among groups (CTL, LI, HI) were only observed between control_T1 and HI_T1 (Table 2). An opposite trend was observed for GSHt, with significantly lower values at the beginning of the experiment (Control_T0, 116.19 ± 18.1 nmol g−1 FW) in comparison to values recorded at HI_T2 (184.79 ± 47.31 nmol g−1 FW) (Table 2). Nevertheless, no significant differences were observed among conditions at each sampling period. In CAT activity, results showed no significant differences among groups from the same sampling time and among sampling periods for the same group (Table 2). Finally, regarding the SOD activity, no significant differences were observed among groups for each sampling period, but some differences were found between LI clams from T1 and T2 (Table 2).
Table 2. Values of the different oxidative stress parameters are expressed in mean ± standard deviation
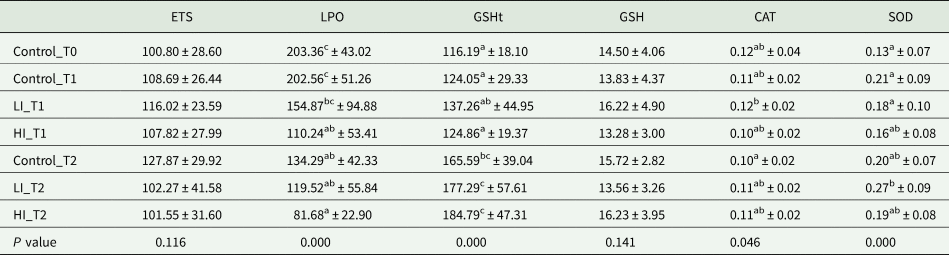
ETS is presented in nmol min−1 g−1 FW; LPO in nmol MDA g−1 FW; GSH and GSHt in nmol g−1 FW while CAT and SOD are expressed in U mg−1 protein. Significant differences among groups are indicated with superscript letters (P < 0.05).
Spawning and larvae viability
Two days after induction of spawning, 12.8 × 106 larvae were obtained from control group clams, 3.02 × 106 larvae from LI and 3.55 × 106 from the HI group. High mortality of larvae was observed in each group on day 6 post-spawning; this was especially intense in the LI group with a 98.41% of mortality, while larvae from the control and HI group presented 31.67 and 78.37%, respectively (Fig. 6).

Fig. 6. Number of larvae (in millions) obtained at days 2 and 6 after spawning of clams from each group after 60 days of experiment.
Discussion
In the present paper, the effect of P. olseni on clam gametogenesis and fecundity was evaluated using several morphometric and biochemical tools. The injection protocol successfully provoked an intense accumulation of parasite cells in clam's gills and the level of infection remained stable during the 2-month trial. These results are in accordance with those previously obtained by Garcia et al. (Reference Garcia, Estêvão, Costas, Cruz and Fernández-Boo2022) in which a moderate infection was measured in the LI group and a high infection in the HI group but without an increase of parasite cells during time. It was previously documented, that unlike juvenile clams, R. philippinarum adults are able to avoid a rapid proliferation of Perkinsus cells while maintaining a stable level of infection during time (Waki et al., Reference Waki, Shimokawa, Watanabe, Yoshinaga and Ogawa2012) which seems that is happening here. The results showed that significantly higher mortality occurred in both infected groups in comparison with control. This result is incongruent with previous infection studies using the same methodology where no-significant mortality was observed using similar treatments and similar water temperature. These results suggest that animals were potentially in a weaker physiological condition than in the previous experiment. Although the animals came from the same area, the collection was made in a different season that could imply a different physiological condition and could have negative effect on survival.
Some differences were also found in the measure of stress parameters. Although no differences were found in SOD activity, a reduction of the detrimental effects on cell membranes when compared to controls (measured through LPO determination) was clear, especially in HI group. The limited ETS activity in infected clams compared with control ones could be associated with limited reactive oxigen species (ROS) production, explaining low LPO levels in the most stressful conditions, even under low antioxidant capacity. Here, organisms may be experiencing a limited antioxidant capacity resulting from an overall reduction in clams’ metabolism, which is a common strategy associated with reduced filtration and respiration capacities, a behaviour frequently observed in bivalves exposed to stressful conditions (Lassudrie et al., Reference Lassudrie, Soudant, Richard, Henry, Medhioub, da Silva, Donval, Bunel, Le Goïc, Lambert, de Montaudouin, Fabioux and Hégaret2014; Magalhães et al., Reference Magalhães, de Montaudouin, Figueira and Freitas2018). Nevertheless, while CAT presented low activity with no clear pattern, we hypothesize that, although without any metabolic activation, the Glutathione peroxidase (GPx) alternative pathway was used to successfully prevent oxidative stress in infected clams using reduced glutathione (GSH) as a co-factor. Reduced glutathione levels did not differ significantly between treatments, but total glutathione content (composed by SH equivalents: GSH + GSSG) was significantly higher in infected clams compared to those not exposed to P. olseni and was also significantly higher over time (from T0 to T2). This trend reveals an increase in pro-oxidant status, i.e. lower GSH/GSSG ratio due to increased oxidased glutathione (GSSG) content, mainly in infected clams at the end of the experiment, which is consistent with the increasingly challenging status. Although the loss of redox balance was observed, especially in infected clams, no cellular damage was noticed.
CI is generally considered to reflect reproductive activity, energy storage and exploitation strategy of bivalve species (Massapina et al., Reference Massapina, Joaquim, Matias and Devauchelle1999; Ojea et al., Reference Ojea, Pazos, Martínez, Novoa, Sánchez and Abad2004; Joaquim et al., Reference Joaquim, Matias, Lopes, Arnold and Gaspar2008). In this study, the maximum values were found before infection, when clams were in sexual rest and the energetic reserves, in glycogen form, were higher. Glycogen is the main energy source stored in gonads as well as other tissues as an energetic reserve for gametogenesis and gonadal development in adult clams (Matias et al., Reference Matias, Joaquim, Matias, Moura, de Sousa, Sobral and Leitão2013). This observation demonstrated that clams were in the best physiological condition to initiate sexual maturation. In the first month of maturation, these values substantially decreased both in non-infected and infected groups, which reflected the advance of gonadal development and the consumption of glycogen reserves. At the end of the first month of the experience, most of the clams were already in full maturation in all conditions and a partial spawning started to occur. However, infected groups showed different behaviours being the control group the more advanced one. Otherwise, clams under high infection had a significant higher CI and showed higher variability in gonadal maturation stages than control and low infected clams, which can be a consequence of a delay in gonadal development, highlighting the effect of the Perkinsus infection on conditioning. This observation is consistent with the results obtained by Casas and Villalba (Reference Casas and Villalba2012) where Perkinsus spp. infection was linked to delays in the gametogenesis in R. decussatus. Similar results were also obtained in oysters infected with P. marinus with a delay in the gametogenesis of infected animals in comparison with non-infected ones (Dittman et al., Reference Dittman, Ford and Padilla2001). During the maturation, the inverse relationship was found between decreasing glycogen and increasing total lipid values, reflecting the biosynthesis of glycogen in lipids during the formation of gametes (Matias et al., Reference Matias, Joaquim, Matias, Moura, de Sousa, Sobral and Leitão2013).
Finally, regarding fecundity, parasite-infected clams seem to produce a lower quantity of gametes due to the lower quantity of larvae obtained at day 2 post-spawning, which is consistent with the results obtained by Park et al. (Reference Park, Figueras and Choi2006) in R. philippinarum clams infected by P. olseni who demonstrated that infected individuals have a lower egg production capacity. Also, the higher mortality rate of larvae from the infected treatments on day 2 could be linked to a lower quantity of neutral lipid reserves in the eggs, which are the main source of energy for the larvae during the first 2 days of life, at the brief endotrophic phase of development (Matias et al., Reference Matias, Joaquim, Ramos, Sobral and Leitão2011). But this theory should be confirmed in future experiments where the lipid content of the eggs should be evaluated between infected and non-infected clams.
Conclusions
During the maturation period (2-month), no differences were found between low and high infected treatments concerning mortality, gonadal development and spawning efficiency, suggesting that even moderate infections could lead to a significant impact on natural populations. In this context, R. decussatus natural beds affected by P. olseni may experience decreased recruitment due to lower-quality larvae derived from infected animals, which can be detrimental to natural populations, especially if this problem is associated with other factors such as environmental changes, contamination or the introduction of new species with better performance. Additionally, the use of infected broodstock for seed production in hatcheries could lead to a lower productivity of the facility given that a delay in sexual maturity, a reduction in fecundity and lower larval viability were observed.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article. Raw data were generated from this study were generated at CIIMAR, University of Aveiro, IPMA and Oceano Fresco S.A. Derived data supporting the findings of this study are available from the corresponding author SFB on request.
Acknowledgements
The authors acknowledge Confradía de Pescadores San Bartolameu de Noia (Galicia, Spain) and especially Liliana Solís for their help in the collection. The authors also thank the following Oceano Fresco hatchery team members for their assistance during the realization of the challenge: Ana Cerviño-Otero, Óscar Iglesias Baños, Ana Torres, Paulo Frias, Carlos Silva and Ricardo Luis.
Financial support
This article is a result of the project ‘INOVAmêijoa: Da maternidade ao viveiro: Inovação no cultivo integral de amêijoa-boa (R. decussatus) e amêijoamacha (V. corrugata)’ ref: CENTRO-01-0247-FEDER-047018/ ALG-01-0247-FEDER-047018, supported by Centro Regional Operational Programme (Centro 2020) and Algarve Regional Operational Programme (CRESC Algarve 2020), under the Portugal 2020 Partnership Agreement, through the European Regional Development Fund (ERDF). This work was also supported by the project Tools4Breed with reference FA_05_2017_025, co-financed by Fundo Azul and Minister of the Sea of the Portuguese Republic, by the project ‘EUROCLAM – Unlocking the potential of Euro-native bivalves’ (CENTRO-01-0247-FEDER-113950) co-financed by CENTRO 2020, PT2020 and ERDF and also by the project BISPECIAl: BIvalveS under Polluted Environment and ClImate chAnge (PTDC/CTA-AMB/28425/2017) funded by FEDER, through COMPETE2020 – Programa Operacional Competitividade e Internacionalização (POCI), and by national funds (OE), through FCT/MCTES. This research was also supported by national funds ‘through FCT – Foundation for Science and Technology’ within the scope of UIDB/0443/2020 and UIDP/04423/2020. Sergio Fernández-Boo was financed by the FCT Scientific Employment Stimulus programme with reference CEECINST/00027/2021. Thanks are also due to FCT/MCTES for the financial support to CESAM (UIDP/50017/2020; UIDB/50017/2020; LA/P/0094/2020) through national funds.
Conflict of interest
None.
Ethical standards
Not applicable.