Article contents
Does Opisthorchis viverrini circulate between humans and domestic cats in an endemic area in Thailand?
Published online by Cambridge University Press: 10 May 2022
Abstract
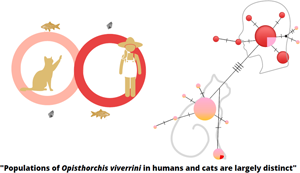
The liver fluke Opisthorchis viverrini is a foodborne trematode that, in chronic infection, is a leading cause of bile-duct cancer – cholangiocarcinoma. Cats and dogs are acknowledged as reservoir hosts of this parasite. However, this assumption is based on morphological similarity of flukes recovered from these hosts, without any molecular genetic evidence. The aim of this study was to obtain molecular data from O. viverrini eggs present in feces of humans and cats in the same locality in Thanya sub-district, Kalasin, Thailand. The mitochondrial cytochrome c oxidase subunit 1 (cox1) gene was used as the marker for a population-genetic study. A DNA fragment of the cox1 gene was amplified from stool samples and subjected to nucleotide sequencing. Phylogenetic and haplotype network analyses were performed. The cox1 sequences of O. viverrini eggs from humans and cats largely formed separate clades on the phylogenetic trees, with an Fst value of 0.64 (P < 0.05), indicating largely distinct populations in the 2 species. However, 5 samples from cats were placed in the human cluster and 1 sample from a human was placed in the cat cluster. This suggests that host specificity of ‘human’ and ‘cat’ clades is not absolute. These results indicate that there are 2 populations of O. viverrini, one circulates primarily in humans and the other in cats. However, cross-transmission can occur between these 2 hosts. Taken altogether, the population-genetic evidence from this study partially supports the assumption that the cat can act as a reservoir host of O. viverrini.
- Type
- Research Article
- Information
- Parasitology , Volume 149 , Special Issue 10: Foodborne Trematodes – time to rise from neglected status , September 2022 , pp. 1334 - 1338
- Copyright
- Copyright © The Author(s), 2022. Published by Cambridge University Press
References
- 10
- Cited by



