Published online by Cambridge University Press: 10 November 2021
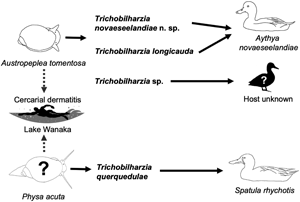
In response to annual outbreaks of human cercarial dermatitis (HCD) in Lake Wanaka, New Zealand, ducks and snails were collected and screened for avian schistosomes. During the survey from 2009 to 2017, four species of Trichobilharzia were recovered. Specimens were examined both morphologically and genetically. Trichobilharzia querquedulae, a species known from four continents, was found in the visceral veins of the duck Spatula rhynchotis but the snail host remains unknown. Cercaria longicauda [i.e. Trichobilharzia longicauda (Macfarlane, 1944) Davis, 2006], considered the major aetiological agent of HCD in Lake Wanaka, was discovered, and redescribed from adults in the visceral veins of the duck Aythya novaeseelandiae and cercariae from the snail Austropeplea tomentosa. Recovered from the nasal mucosa of Ay. novaeseelandiae is a new species of Trichobilharzia that was also found to cycle naturally through Au. tomentosa. Cercariae of a fourth species of Trichobilharzia were found in Au. tomentosa but the species remains unidentified.