Introduction
Small mammals, and particularly rodents, play an integral role in ecosystems, serving as both secondary consumers of seeds and other plant material (Heithaus, Reference Heithaus1981) and as a food resource for various raptors and mesopredators (Preston, Reference Preston1990; Mahmood et al., Reference Mahmood, Niazi and Nadeem2013). The Rodentia is the largest mammalian order, and species within this order have diverse biological and behavioural characteristics. Characteristics such as social structure, habitat usage and nest type are important factors that influence the exposure of rodents to ectoparasites (e.g. fleas and ticks). For example, fossorial species (e.g. mole rats) that make complex permanent underground nests generally harbour a high proportion of mites that are associated with soil and the host nest (Archer et al., Reference Archer, Bennett, Ueckermann and Lutermann2014; Lutermann et al., Reference Lutermann, Carpenter-Kling, Ueckermann, Gutjahr and Bennett2015, Reference Lutermann, Archer, Ueckermann, Junker and Bennett2020). Similarly, arboreal species (e.g. tree squirrels) that have limited contact with the soil surface tend to have few or no ticks which are associated with grass and low-lying vegetation (Patrick and Wilson, Reference Patrick and Wilson1995; Romeo et al., Reference Romeo, Pisanu, Ferrari, Basset, Tillon, Wauters, Martinoli, Saino and Chapuis2013). Opportunism (adaptability) is another characteristic that can influence the parasite profile of a host species. Opportunistic rodent species often have large numbers of parasites due to the fact that they occupy larger geographic areas, often covering multiple vegetation types, and are able to effectively inhabit diverse land-use types (e.g. natural and transformed areas) at a local scale (Feliu et al., Reference Feliu, Renaud, Catzeflis, Hugot, Durand and Morand1997; Lindenfors et al., Reference Lindenfors, Nunn, Jones, Cunningham, Sechrest and Gittleman2007). The presence of rodents and their parasites in anthropogenic areas not only provides a food security risk (Muteka et al., Reference Muteka, Chimimba and Bennett2006) but also creates opportunities for parasite transmission and disease risk in domestic animals and humans (Lecompte et al., Reference Lecompte, Fichet-Calvet, Daffis, Koulémou, Sylla, Kourouma, Doré, Soropogui, Aniskin, Allali, Kan, Lalis, Koivogui, Günther, Denys and Ter Meulen2006; Brettschneider et al., Reference Brettschneider, Bennett, Chimimba and Bastos2012; Mayamba et al., Reference Mayamba, Byamungu, Leirs, Moses, Makundi, Kimaro, Massawe, Kifumba, Nakiyemba, Mdangi, Isabirye and Mulungu2021). It is therefore important that parasite profiles are developed for commensal rodent species as this may identify potential disease-risk areas and facilitate sustainable disease surveillance.
South Africa is known for its rich biodiversity that includes rodents (Skinner and Chimimba, Reference Skinner and Chimimba2005). Micaelamys namaquensis (Namaqua rock mouse, previously Aethomys namaquensis) is a locally abundant and regionally widespread rodent species that is often associated with natural and anthropogenic areas (Withers et al., Reference Withers, Louw and Henschel1980; Skinner and Chimimba, Reference Skinner and Chimimba2005; Starik et al., Reference Starik, Mbango, Bengsch, Göttert and Zeller2020). The rodent occurs throughout South and southern Africa (Skinner and Chimimba, Reference Skinner and Chimimba2005; Apps, Reference Apps2012; Monadjem et al., Reference Monadjem, Taylor, Denys and Cotterill2015). However, recent phylogeographic studies on M. namaquensis suggest biome-associated molecular differentiation in South Africa (Chimimba, Reference Chimimba2001; Russo et al., Reference Russo, Chimimba and Bloomer2010; Bothma et al., Reference Bothma, Matthee and Matthee2021). Though the species can inhabit a wide variety of biomes, it distinctly prefers rocky substrates such as outcrops, boulders and hillsides (Skinner and Chimimba, Reference Skinner and Chimimba2005; Fagir et al., Reference Fagir, Ueckermann, Horak, Bennett and Lutermann2014). Micaelamys namaquensis often uses dry grass to construct nests in rock crevices and overhangs as well as occasionally in hollows of trees (Roberts, Reference Roberts1951; Ansell, Reference Ansell1960; Choate, Reference Choate1972). The species feeds on seeds, green plant material and insects (Woodall and Mackie, Reference Woodall and Mackie1987; Kerley et al., Reference Kerley, Knight and Erasmus1990; Monadjem, Reference Monadjem1997). Micaelamys namaquensis is described as social (Skinner and Chimimba, Reference Skinner and Chimimba2005) and makes communal nests (Choate, Reference Choate1972; Flemming and Nicolson, Reference Flemming and Nicolson2004).
As yet, little is known about the ectoparasite species associated with naturally occurring rodents in South Africa. Though parasite–host and host–parasite lists are available for most rodent taxa (e.g. Zumpt, Reference Zumpt1961; Theiler, Reference Theiler1962; Ledger, Reference Ledger1980; Segerman, Reference Segerman1995; Horak et al., Reference Horak, Heyne, Williams, Gallivan, Spickett, Bezuidenhout and Estrada-Peña2018) the data are often outdated and in need of revision (van der Mescht and Matthee, Reference van der Mescht and Matthee2017). The main critique of these reports and monographs includes incomplete sample ranges and inadequate sample sizes (Shvydka et al., Reference Shvydka, Sarabeev, Estruch and Cadarso-Suárez2018; Wilson et al., Reference Wilson, Dobson, Merler, Poglayen, Randolph, Read, Skorping, Hudson, Rizzoli, Grenfell, Heesterbeek and Dobson2001; Stockwell and Peterson, Reference Stockwell and Peterson2002). In addition, at present there is a lack of geographic distribution maps for lice and mite species that are associated with rodents in South Africa. In recent years, empirical studies, based on larger sample sizes, have been conducted on a few rodent species (Matthee et al., Reference Matthee, Horak, Beaucournu, Durden, Ueckermann and McGeoch2007, Reference Matthee, Horak, van der Mescht, Ueckermann and Radloff2010; Hillegass et al., Reference Hillegass, Waterman and Roth2008; Archer et al., Reference Archer, Bennett, Ueckermann and Lutermann2014; Barnard et al., Reference Barnard, Krasnov, Goff and Matthee2015; Lutermann et al., Reference Lutermann, Carpenter-Kling, Ueckermann, Gutjahr and Bennett2015); these include a study on M. namaquensis (Fagir et al., Reference Fagir, Ueckermann, Horak, Bennett and Lutermann2014). Although this is a step in the right direction, these studies are limited in extent as they are restricted to a single locality and/or biome. Based on current literature, M. namaquensis acts as a host for numerous ectoparasite taxa (Zumpt, Reference Zumpt1961; Theiler, Reference Theiler1962; Ledger, Reference Ledger1980; Segerman, Reference Segerman1995; Fagir et al., Reference Fagir, Ueckermann, Horak, Bennett and Lutermann2014; Horak et al., Reference Horak, Heyne, Williams, Gallivan, Spickett, Bezuidenhout and Estrada-Peña2018) and is also a reservoir host for vector-borne pathogens such as Bartonella (Brettschneider et al., Reference Brettschneider, Bennett, Chimimba and Bastos2012). Given the wide distribution and opportunistic behaviour of M. namaquensis, it is predicted that the ectoparasite diversity is currently underestimated. This prediction is supported by a recent country-wide study on flea species associated with murid rodents (van der Mescht and Matthee, Reference van der Mescht and Matthee2017). To address this paucity in data, M. namaquensis was sampled and the ectoparasites were recorded at multiple localities and across several biomes within its distribution range in South Africa. The aim of the study was to quantify the species richness and infestation parameters of ectoparasites associated with M. namaquensis in South Africa. In addition, by combining the results from the current study with those of previous studies, the study aimed to provide preliminary distribution maps for the lice and more common mite species that occur on M. namaquensis and other rodent species in South Africa. Lastly, the development of a comprehensive ectoparasite species list provides information on the importance of M. namaquensis as a host for ectoparasite species of which some species are of veterinary and medical importance.
Materials and methods
The ectoparasite material used in the study was obtained from a previous study conducted on the molecular ecology of sucking lice (Anoplura) associated with the Aethomys/Micaelamys rodent complex (Bothma et al., Reference Bothma, Matthee and Matthee2020, Reference Bothma, Matthee and Matthee2021).
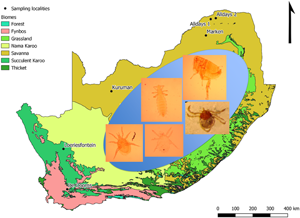
Micaelamys namaquensis individuals (n = 216) were trapped at 12 localities across South Africa during austral summer, autumn and winter in 2017 and 2018. The localities represented several biomes: Fynbos (1), Grassland (3), Savanna (7) and Succulent Karoo (1) (Fig. 1; Table 1). Sampling was conducted using Sherman-like live traps that were baited using a mixture of peanut butter and oats. Traps were set for 2–4 days per locality during which time they were checked twice per day. The morphological identification of M. namaquensis was confirmed molecularly using mitochondrial cytochrome oxidase subunit I (Bothma et al., Reference Bothma, Matthee and Matthee2020; S. Matthee, unpublished data).

Fig. 1. Sampling localities (n = 12) and biomes where Micaelamys namaquensis (n = 216) were trapped in South Africa during 2017–2018.
Table 1. Locality information, sample size per locality and sampling period for Micaelamys namaquensis (n = 216) trapped in South Africa during 2017–2018

Only adult M. namaquensis (> 30 g) were included in the study. Captured individuals were placed in separate plastic bags along with a reference number and euthanized with 2–4 mL sodium pentobarbitone (200 mg kg−1) depending on individual body weight. After euthanasia, each individual was weighed and frozen at −20°C in the field and transferred to a −80°C freezer in the laboratory. Prior to parasite removal, the frozen carcasses were thawed and systemically examined under a stereoscopic microscope. All fleas, lice, mesostigmatid mites and ticks were removed, while only a subsample of larval trombiculid mites (chiggers) were removed with forceps. Ectoparasite taxa were placed in individual sample tubes containing 70% ethanol. Fleas, lice, mesostigmatid mites (hereafter referred to as mites) and chiggers were cleared, and the slide mounted using standard techniques, while ticks remained unmounted.
Ectoparasite identification was conducted using taxonomic reference keys. Fleas were identified according to Segerman (Reference Segerman1995). Lice were sorted into morphospecies and a subsample from each locality was slide mounted and identified using various reference sources (e.g. Johnson, Reference Johnson1960; Kleynhans, Reference Kleynhans1969; Ledger, Reference Ledger1980; Durden and Musser, Reference Durden and Musser1994). Two congeneric louse species, Hoplopleura patersoni and Hoplopleura aethomydis, share several morphological characteristics making differentiation between the 2 species troublesome. For this study the specimens were identified as H. cf. patersoni, as they share many morphological characters. However, the type specimens of these species will need to be studied to confirm whether they are indeed 2 distinct species. Due to technical difficulties not all the nymphs of the 2 lice species (H. cf. patersoni and Polyplax praomydis) could be identified, thus they were pooled and presented as undifferentiated nymphs. Given these restrictions, all calculations for lice species were based only on the adult stage. Mesostigmatid mites were identified according to Herrin and Tipton (Reference Herrin and Tipton1976) and Krantz and Walter (Reference Krantz and Walter2009). The larval stage of mites in the family Trombiculidae (referred to as chiggers) was identified following Stekolnikov (Reference Stekolnikov2018) and the extensive taxonomic literature referenced to therein. In addition, the parasitope (body region) of the chiggers on the host was recorded at 2 localities. Ticks were identified using various reference sources (e.g. Walker et al., Reference Walker, Keirans and Horak2000; Horak et al., Reference Horak, Heyne, Williams, Gallivan, Spickett, Bezuidenhout and Estrada-Peña2018). Conclusive species identifications were not possible for several ticks, notably immature stages, and they were subsequently allocated to either species groups or unknown species within a relevant genus.
The calculation of mean abundance and prevalence followed the guidelines provided in Bush et al. (Reference Bush, Lafferty, Lotz and Shostak1997). The mean parasite abundance was calculated as the total number of individuals of a particular species divided by the total number of hosts examined regardless of parasite presence. Species prevalence was calculated by recording the total number of hosts that had 1 or more individuals of a particular species present divided by the total number of hosts examined. The per-locality mean abundance and prevalence of each taxon and species were calculated similarly but the samples were restricted to these localities. Unless otherwise stated, mean abundance is presented as the mean value ± standard error. Species richness for individual parasite taxa was determined by counting the number of species present at a given locality for the parasite taxon. Only prevalence data are available for chiggers. As the chigger parasitope was not consistently recorded throughout, parasitope preference was only calculated for 2 localities (Bloemfontein and Kimberley) where the parasitope for all or the majority of samples was reported. Parasitope preferences were calculated based on chigger presence/absence on a parasitope and were reported as a percentage of chigger infestation.
Results
A total of 5591 ectoparasites (fleas, lice, mesostigmatid mites and ticks, excluding chiggers) was recorded from 216 M. namaquensis individuals. At least 57 ectoparasite taxa (represent species and species groups), representing at least 26 genera, were identified (Table 2). Ticks were the most speciose taxon (20 taxa), followed by chiggers (14 species), mesostigmatid mites (11 taxa) and fleas (10 species), while lice were the most prevalent (71.76%) followed by mesostigmatid mites (50.93%) (Table 3).
Table 2. Ectoparasite taxa collected from Micaelamys namaquensis (n = 216) trapped across South Africa during 2017–2018

Table 3. Infestation parameters for the ectoparasite taxa recorded on Micaelamys namaquensis (n = 216) in South Africa during 2017–2018

Bold text indicate the values at the higher taxonomic level.
a Data based on adult life stage only.
Ten flea species, representing 6 genera, were recorded on M. namaquensis (Table 2). Overall, Xenopsylla brasiliensis was the most abundant and prevalent species (0.89 ± 0.13, 28.24%, respectively) followed by Chiastopsylla godfreyi (0.53 ± 0.17, 10.65%) (Table 3). Xenopsylla brasiliensis was recorded at 7 localities (Table 4) and was present on ≥10% of the rodents at each locality (Table 5). Chiastopsylla godfreyi and Dinopsyllus ellobius were more restricted in their distribution (4 localities) and less prevalent compared to X. brasiliensis (Tables 4 and 5). Female-biased flea infestations were only recorded for 3 species (Chiastopsylla octavii, Ctenocephalides felis and Epirimia aganippes) (Table 3), while male-biased sex ratios were recorded for C. godfreyi and X. brasiliensis at several localities (Supplementary Table 1).
Table 4. Mean abundance (±s.e.) and occurrence per locality of individual ectoparasite taxa recovered from Micaelamys namaquensis (n = 216) across South Africa during 2017–2018

a Data based on adult life stage only.
Table 5. Per locality prevalence (%) of individual ectoparasite taxa recovered from Micaelamys namaquensis (n = 216) across South Africa during 2017–2018

a Data based on adult life stage only.
Two louse species, H. cf. patersoni and P. praomydis, were recorded (Table 2). The following data are based on adult stages only. The overall mean abundance and prevalence of H. cf. patersoni were lower (2.94 ± 0.46, 43.06%) than P. praomydis (7.48 ± 1.11, 47.69%) (Table 3). Hoplopleura cf. patersoni was present at 10 localities and P. praomydis at 9, while the 2 species co-occurred at 7 of the 12 localities (Table 4). Apart from an outlier (Alldays 2), P. praomydis was collected at the central and western localities, while H. cf. patersoni was recovered at the central and north-eastern localities (Fig. 2). Polyplax praomydis was more abundant and prevalent than H. cf. patersoni at 5 of the 6 central localities where they co-occurred (Tables 4 and 5). In both species the sex ratio was variable between localities (Supplementary Table 2).

Fig. 2. Occurrence records (n = 14) for Hoplopleura cf. patersoni and Polyplax praomydis recorded from Micaelamys namaquensis in South Africa (data from the present study; Fagir et al., Reference Fagir, Ueckermann, Horak, Bennett and Lutermann2014; Bothma et al., Reference Bothma, Matthee and Matthee2020, Reference Bothma, Matthee and Matthee2021).
A total of 11 mesostigmatid mite species was collected from M. namaquensis (Table 2). Androlaelaps rhabdomysi was the most abundant and prevalent species (1.22 ± 0.28, 25.46%) followed by Laelaps fritzumpti (0.80 ± 0.14, 24.07%) (Table 3). Androlaelaps rhabdomysi was recorded at 9 localities and L. fritzumpti at 8 (Table 4). Androlaelaps rhabdomysi was present across South Africa, while L. fritzumpti occurred in the central and north-eastern localities (Fig. 3). Female-biased infestations were recorded for 10 mite species (Table 3) and this pattern was common across localities (Supplementary Table 3).

Fig. 3. Occurrence record (n = 14) for Androlaelaps rhabdomysi and Laealaps fritzumpti recorded from Micaelamys namaquensis and Rhabdomys pumilio (data from the present study; Matthee and Ueckermann, Reference Matthee and Ueckermann2008; Fagir et al., Reference Fagir, Ueckermann, Horak, Bennett and Lutermann2014; S. Matthee, unpublished data).
A total of 14 chigger species was recorded from M. namaquensis (Table 2). The chiggers represent 10 known species, 2 new undescribed species (Kayella sp. and Schoutedenichia sp.) and 2 potentially new species that require additional examination (Hyracarus sp. and Hyracarus aff. namibiensis). New localities were recorded for 5 chigger species. Only 2 species occurred at multiple localities: H. aff. namibiensis was present at 4 localities, followed by Schoutedenichia morosi at 3 localities (Table 5). The per-locality species richness varied among localities with 5 species recorded at Steynsburg followed by 3 at Alldays 2 (Table 5). The per-locality prevalence of chiggers varied between 7.69% (Alldays 2) and 77.78% (Bethulie). Chiggers were recorded from 6 parasitopes on the host (body, ear, genital area, head, leg and tail base) of which the ear was one of the preferred parasitopes at both Bloemfontein (71.43%) and Kimberley (72.73%) (Table 6). In addition, the tail base was also one of the preferred parasitopes (71.43 and 27.27%, respectively) (Table 6).
Table 6. Summary of parasitope preference, presented as prevalence (%), for chiggers recovered from Micaelamys namaquensis (n = 41) at 2 localities in South Africa during June 2018

Ticks were represented by larvae (74.95%) and nymphs (24.66%), while adult stages were only recorded for Ixodes bakeri-like ticks. Five tick genera of which 12 species and 8 species groups (including unknown species) were recovered on M. namaquensis (Table 2). The most abundant and prevalent ticks belonged to 2 species groups Rhipicephalus simus/follis (0.70 ± 0.23, 11.11%) and Haemaphysalis spinulosa group (0.58 ± 0.20, 15.74%) (Table 3). While the most abundant tick species was Rhipicephalus neumanni (0.24 ± 0.14) and Haemaphysalis elliptica was the most prevalent (5.56%), Rhipicephalus follis was the most widespread (occurred at 4 localities), while the remaining tick species were recorded at fewer localities (1–3 localities) (Tables 4 and 5). A single individual of Nuttalliella cf. namaqua was recorded at 1 locality (Loeriesfontein).
Discussion
The study recorded a large diversity of ectoparasites that include 57 ectoparasite taxa (49 species and 8 species groups). This includes several new locality and host records for ectoparasite taxa and reveals the existence of undescribed species. This finding supports the prediction that the current ectoparasite profile of M. namaquensis is underestimated. The large diversity is possibly attributed to the opportunistic behaviour of M. namaquensis (Macdonald et al., Reference Macdonald, Mathews, Berdoy, Singleton, Hinds, Leirsand and Zhang1999; Soliman et al., Reference Soliman, Marzouk, Main and Montasser2001). A similar pattern was recorded for Rhabdomys pumilio (4-striped mouse), another opportunistic rodent species in South Africa (Matthee et al., Reference Matthee, Horak, Beaucournu, Durden, Ueckermann and McGeoch2007, Reference Matthee, Horak, van der Mescht, Ueckermann and Radloff2010; Froeschke et al., Reference Froeschke, van der Mescht, McGeoch and Matthee2013; Barnard et al., Reference Barnard, Krasnov, Goff and Matthee2015; van der Mescht and Matthee, Reference van der Mescht and Matthee2017). In particular, >30 ectoparasite taxa (representing fleas, lice, mesostigmatid and trombiculid mites and ticks) are associated with R. pumilio in the southern and western parts of South Africa (Matthee et al., Reference Matthee, Horak, Beaucournu, Durden, Ueckermann and McGeoch2007, Reference Matthee, Horak, van der Mescht, Ueckermann and Radloff2010; Froeschke et al., Reference Froeschke, van der Mescht, McGeoch and Matthee2013; Barnard et al., Reference Barnard, Krasnov, Goff and Matthee2015; van der Mescht and Matthee, Reference van der Mescht and Matthee2017). Fagir et al. (Reference Fagir, Ueckermann, Horak, Bennett and Lutermann2014) recorded at least 22 ectoparasite taxa on M. namaquensis at a single locality in the Savanna biome in South Africa. The latter study was based on 313 M. namaquensis individuals trapped seasonally over 12 months.
In the present study, fleas were present on almost 50% of the rodents. Xenopsylla brasiliensis and C. godfreyi were the most prevalent and abundant flea species, supporting the findings of Fagir et al. (Reference Fagir, Ueckermann, Horak, Bennett and Lutermann2014). Both flea species were characterized by male-biased infestation at most (C. godfreyi), or all localities (X. brasiliensis). The sex-ratio pattern recorded for X. brasiliensis is supported by de Meillon et al. (Reference de Meillon, Davis and Hardy1961) and Fagir et al. (Reference Fagir, Ueckermann, Horak, Bennett and Lutermann2014). However, the pattern recorded for C. godfreyi is not supported by previous records (de Meillon et al., Reference de Meillon, Davis and Hardy1961; Fagir et al., Reference Fagir, Ueckermann, Horak, Bennett and Lutermann2014) and warrants further research. According to previous records (Segerman, Reference Segerman1995; Fagir et al., Reference Fagir, Ueckermann, Horak, Bennett and Lutermann2014; van der Mescht and Matthee, Reference van der Mescht and Matthee2017), C. godfreyi, E. aganippes and Listropsylla aricinae should have a close association with M. namaquensis. However, the low occurrence of L. aricinae may be due to an incomplete overlap between the current sampling localities and the flea's preferred geographic distribution (spanning the drier western part of South Africa from the Cape to Namibia) (Segerman, Reference Segerman1995). Four generalist (i.e. broader host preferences) flea species (D. ellobius, Listropsylla agrippinae, Listropsylla dorippae and X. brasiliensis) were abundant on M. namaquensis in the present study. These fleas are known vectors of plague (causative agent Yersinia pestis). Dinopsyllus ellobius has been identified as one of the most significant plague vectors in South Africa (Ingram, Reference Ingram1927; de Meillon et al., Reference de Meillon, Davis and Hardy1961) and both Dinopsyllus lypusus (also recorded in the study) and L. dorippae are important and efficient vectors for plague in Africa (Heisch et al., Reference Heisch, Grainger and D'Souza1953; Kilonzo and Mhina, Reference Kilonzo and Mhina1982, Reference Kilonzo and Mhina1983; Makundi et al., Reference Makundi, Kilonzo, Massawe, Singleton, Hinds, Krebs and Spratt2003). Ctenocephalides felis is a reservoir and vector of Rickettsia felis, which can be transmitted to both humans and animals, including domestic cats (Harasen and Randall, Reference Harasen and Randall1986; Greene and Breitschwerdt, Reference Greene, Breitschwerdt and Greene2006; Tsai et al., Reference Tsai, Lu, Huang, Wang, Wang, Huang, Wu and Shu2009) and this flea can transmit Bartonella spp. (Bouhsira et al., Reference Bouhsira, Ferrandez, Liu and Franc2013). Transmission of Bartonella is mainly through the feces of infected fleas (Foil et al., Reference Foil, Andress, Freeland and Roy1998; Finkelstein et al., Reference Finkelstein, Brown, O'Reilly and Wedincamp2002; Gutiérrez et al., Reference Gutiérrez, Krasnov, Morick, Gottlieb, Khokhlova and Harrus2015).
The 2 louse species recorded on M. namaquensis, H. cf. patersoni and P. praomydis, are both known from M. namaquensis and the closely related Aethomys chrysophilus (red rock rat) (Durden and Musser, Reference Durden and Musser1994; Braack et al., Reference Braack, Horak, Jordaan, Segerman and Louw1996; Fagir et al., Reference Fagir, Ueckermann, Horak, Bennett and Lutermann2014). These host associations are based on morphological characters, although a recent molecular study provides strong evidence that H. cf. patersoni on M. namaquensis is genetically distinct from the same morphotype on A. chrysophilus (Bothma et al., Reference Bothma, Matthee and Matthee2020). It is quite possible that the same holds true for P. praomydis, but this remains to be tested (Bothma et al., Reference Bothma, Matthee and Matthee2020). Cryptic species have been detected in several ectoparasite taxa (Poulin and Keeney, Reference Poulin and Keeney2008; Malenke et al., Reference Malenke, Johnson and Clayton2009), including the louse Polyplax arvicanthis on the widely distributed Rhabdomys genus in South Africa (du Toit et al., Reference du Toit, Matthee and Matthee2013). The presence of cryptic species among parasites may be a common occurrence as their small body size and morphological stages can cause some difficulty in distinguishing between species based on morphology (Perkins et al., Reference Perkins, Martinsen and Falk2011). Overall, lice were the most prevalent taxon and showed the highest mean abundance of all the ectoparasite taxa in the present study. Both louse species showed greater prevalence and mean abundance on M. namaquensis in the present study (43.06%, 2.94 ± 0.46 and 47.69%, 7.48 ± 1.11, respectively) compared to Fagir et al. (Reference Fagir, Ueckermann, Horak, Bennett and Lutermann2014) (16.2%, 0.89 ± 0.27 and 2.1%, 0.10 ± 0.04). These differences may be due to variation (in study design and parasite removal methods) between the 2 studies. As mentioned, the study by Fagir et al. (Reference Fagir, Ueckermann, Horak, Bennett and Lutermann2014) was conducted seasonally at 1 locality and in addition rodents were visually inspected. In contrast, whole-body examinations were conducted under a stereomicroscope in the present study. Female-biased sex ratios were not a general pattern for either of the 2 louse species. It is possible that louse sex ratios vary seasonally and in association with individual louse infestations on the host. The latter has been recorded for anoplurid lice on humans (Rozsa, Reference Rozsa1997) and chewing lice on birds (Rozsa et al., Reference Rozsa, Rekasi and Reiczigel1996; Pap et al., Reference Pap, Adam, Vágási, Benko and Vincze2013). Based on the findings in the present study, the geographic range of the 2 louse species only partially overlaps: H. cf. patersoni occurred in the central and eastern summer rainfall regions of the country, while P. praomydis was more widely distributed across South Africa, which suggests that the latter species is more tolerant of diverse climatic conditions (present in summer and winter rainfall regions).
Mites (in the order Mesostigmata) were recorded on half of all rodents. In all, 3 species, namely A. rhabdomysi, L. fritzumpti and Laelaps aff. grenieri were the most common mite species. The high prevalence of A. rhabdomysi (25.46%) is supported by Fagir et al. (Reference Fagir, Ueckermann, Horak, Bennett and Lutermann2014), who recorded a 20.4% prevalence for A. rhabdomysi on M. namaquensis. Only 2 mite species (A. rhabdomysi and Androlaelaps zuluensis) were shared with Fagir et al. (Reference Fagir, Ueckermann, Horak, Bennett and Lutermann2014). In general, the overall dominance of A. rhabdomysi, L. fritzumpti and L. aff. grenieri, in the present study, was also recorded at the individual localities where they occurred. Androlaelaps rhabdomysi occurred at most of the sampling localities. However, based on the per-locality-infestation levels, it does appear that the species prefers the central localities such as Kimberley, Kuruman, Steynsburg and Bethulie. Previous studies on R. pumilio also recorded this species in the western and southern regions of South Africa, which suggests that the mite species has a country-wide distribution (Matthee and Ueckermann, Reference Matthee and Ueckermann2008; S. Matthee, unpublished data). In the present study, L. fritzumpti was absent from the southern and south-western winter rainfall localities and more common in the summer rainfall central and north-eastern localities. There is also no record of this species on 2 other rodent species [Otomys irroratus (Southern African vlei rat) and R. pumilio] at various localities in the Western Cape Province (Matthee et al., Reference Matthee, Horak, Beaucournu, Durden, Ueckermann and McGeoch2007, Reference Matthee, Horak, van der Mescht, Ueckermann and Radloff2010). Host records for L. fritzumpti include a range of host species such as Aethomys spp., Elephantulus rupestris (western rock sengi), Gerbillurus paeba (hairy-footed gerbil) and R. pumilio (Herrin and Tipton, Reference Herrin and Tipton1976). The third most prevalent mite, L. aff. grenieri, was present at 5 localities, of which 4 were located centrally and 1 in the north-eastern region of South Africa. Laelaps grenieri (Taufflieb and Mouchet, Reference Taufflieb and Mouchet1956) has been recorded from a multitude of small mammal hosts, primarily Lemniscomys spp. in north-western Africa [Democratic Republic of the Congo (DRC); Zumpt, Reference Zumpt1961]. The great geographical distance between the present study and the DRC, as well as morphological inconsistencies between the 2 species, suggests that our mite is different from, but shares resemblances with L. grenieri from the DRC. Female-biased infestations were common for the most abundant mite species (A. rhabdomysi, L. fritzumpti and L. aff. grenieri) and are in agreement with previous studies on South African rodents [M. namaquensis (Fagir et al., Reference Fagir, Ueckermann, Horak, Bennett and Lutermann2014) and R. pumilio (Matthee et al., Reference Matthee, Horak, Beaucournu, Durden, Ueckermann and McGeoch2007)]. The pattern of female bias is a widespread phenomenon among parasitic mites and may be attributed to the parthenogenetic reproductive systems of mites (Norton et al., Reference Norton, Kethley, Johnston, O'Connor, Wrensch and Ebbert1993; Sonenshine, Reference Sonenshine and Sonenshine1993; Matthee et al., Reference Matthee, Horak, Beaucournu, Durden, Ueckermann and McGeoch2007). In addition, female laelapid mites require more frequent blood meals (for oogenesis) and also use hosts to disperse. This is in contrast to males that feed less and remain in the nest (Radovsky, Reference Radovsky and Kim1985, Reference Radovsky and Houck1994). The remaining 8 mesostigmatid mite species appeared to be less common on M. namaquensis and have more localized distributions (present at 1–3 localities). Interestingly, 3 species (A. zuluensis, Laelaps muricola and Laelaps vansomereni) were previously recorded on M. namaquensis (Zumpt, Reference Zumpt1961; Engelbrecht et al., Reference Engelbrecht, Matthee, Ueckermann and Matthee2014). The low occurrence of at least 2 of the remaining species may be due to a preference for other rodent species and/or geographic areas. For example, Androlaelaps dasymys was present on M. namaquensis at 1 locality (Hammanskraal), but this mite has been recorded on R. pumilio and O. irroratus in the southern (Stellenbosch, Somerset West, Malmesbury, Grabouw and Swellendam), western (Vanrynsdorp) and north-western (Springbok and Groblershoop) parts of South Africa (Matthee et al., Reference Matthee, Horak, Beaucournu, Durden, Ueckermann and McGeoch2007, Reference Matthee, Horak, van der Mescht, Ueckermann and Radloff2010; S. Matthee, unpublished data). It is evident from this study that current information with regard to host and geographic range of mesostigmatid mites on rodents is lacking for South Africa.
Chiggers were present on almost half (41.20%) of all rodents. Fourteen species were recorded of which 2 are new undescribed species and 2 are potentially new species. The discovery of new chigger species on rodents in South Africa is mainly due to past limited research interest in the taxonomic group and the lack of local taxonomic expertise (Barnard et al., Reference Barnard, Krasnov, Goff and Matthee2015; Stekolnikov and Matthee, Reference Stekolnikov and Matthee2019). Gahrliepia nana, Microtrombicula mastomyia and S. morosi have previously been recorded on rodents [e.g. Aethomys ineptus (Tete veld rat) and Saccostomus campestris (South African pouched mouse)] in the north-eastern Savanna biome in South Africa (Zumpt, Reference Zumpt1961; Matthee et al., Reference Matthee, Stekolnikov, van der Mescht, Froeschke and Morand2020). Herpetacarus gerrhosauri and Hyracarus longipilosus are known species that are recorded here for the first time after their description on 2 lizard species Gerrhosaurus flavigularis (yellow-throated plated lizard) and Pseudocordylus subviridis (Drakensberg crag lizard) from Mullers Pass and Witzieshoek Naturelle Reserve, Free State, South Africa for the former and Procavia capensis (rock hyrax) from Cedara, KwaZulu-Natal, South Africa for the latter by Lawrence (Reference Lawrence1949). Hypotrombidium meleagride presents the first record outside the type locality, which is Malmesbury in the Western Cape, South Africa (Vercammen-Grandjean and Langston, Reference Vercammen-Grandjean and Langston1976). Microtrombicula squirreli and Schoutedenichia paraxeri are recorded in South Africa for the first time as these species were previously recorded from the DRC (Zumpt, Reference Zumpt1961; Stekolnikov, Reference Stekolnikov2018). The present study provides the first record of the genus Kayella in South Africa. The genus was previously recorded in localities spanning Europe, Asia and North America (Nielsen et al., Reference Nielsen, Robbins and Rueda2021). Lastly, Tateracarus foliosetosus and Tateracarus kimberleyensis are the second and third species of the previously monotypic genus Tateracarus, with the type species Tateracarus quadrisetosus, recorded on Gerbilliscus leucogaster (bushveld gerbil) in Namibia (Stekolnikov, Reference Stekolnikov2018; Stekolnikov and Matthee, Reference Stekolnikov and Matthee2022). In the present study, most chigger species were recorded at a single locality. However, 2 species (H. aff. namibiensis and S. morosi) were recorded at multiple localities. Chiggers are regarded as habitat specialists (Shatrov and Kudryashova, Reference Shatrov, Kudryashova, Morand, Krasnov and Poulin2006) though some species have wider tolerance ranges and can occur in multiple habitat types (Mohr, Reference Mohr1947; Traub and Wisseman, Reference Traub and Wisseman1974; Matthee et al., Reference Matthee, Stekolnikov, van der Mescht, Froeschke and Morand2020). Based on the chigger data from 2 localities in the Savanna biome, the ears were the preferred parasitope on M. namaquensis. This parasitope has previously been recorded for chiggers, as a group, on M. namaquensis in the south-eastern Savanna biome, South Africa (Fagir et al., Reference Fagir, Ueckermann, Horak, Bennett and Lutermann2014; D. Fagir, personal communication). Microtrombicula mastomyia displayed a similar preference for rodent ears in a recent study conducted in the north-eastern Savanna biome in South Africa (Matthee et al., Reference Matthee, Stekolnikov, van der Mescht, Froeschke and Morand2020). The thickness of the host skin may be an important factor as the cheliceral blades that are used for attachment are minute (e.g. 24 and 35 μm for Schoutedenichia horaki and Ascoschoengastia ueckermanni, respectively, from South Africa) (Matthee et al., Reference Matthee, Stekolnikov, van der Mescht, Froeschke and Morand2020). A preference for thin-skinned areas has also been recorded for chiggers on lizards (Arnold, Reference Arnold1982; Goldberg and Holshuh, Reference Goldberg and Holshuh1992; Klukowski, Reference Klukowski2004). In addition, the ear parasitope provides protection against removal by oral grooming (Goff, Reference Goff1979; Barnard et al., Reference Barnard, Krasnov, Goff and Matthee2015). Similar to previous studies, the tail base was also one of the preferred parasitopes in the present study (Barnard et al., Reference Barnard, Krasnov, Goff and Matthee2015; Matthee et al., Reference Matthee, Stekolnikov, van der Mescht, Froeschke and Morand2020).
Ticks were present on half of all rodents and were represented by 20 taxa of which 12 were identified to species and 8 to species groups (including unknown species). In general, immature life stages (larvae and nymphs) were recorded on M. namaquensis (Petney et al., Reference Petney, Horak, Howell and Meyer2004; Durden, Reference Durden, Morand, Krasnov and Poulin2006; Matthee et al., Reference Matthee, Horak, Beaucournu, Durden, Ueckermann and McGeoch2007, Reference Matthee, Horak, van der Mescht, Ueckermann and Radloff2010; Horak et al., Reference Horak, Heyne, Williams, Gallivan, Spickett, Bezuidenhout and Estrada-Peña2018). The morphological characters of larvae and nymphs are such that they can easily be identified to genus level, but these characters are often not sufficiently diagnostic to make a specific diagnosis (Walker et al., Reference Walker, Keirans and Horak2000). As a result, several of the larvae and nymphs were assigned to species groups. The genera, Rhipicephalus and Haemaphysalis represented most of the tick taxa (species and species groups). The higher species richness recorded for Rhipicephalus is supported by Fagir et al. (Reference Fagir, Ueckermann, Horak, Bennett and Lutermann2014), where 6 of the 8 tick taxa were conspecifics within the Rhipicephalus genus. It is not possible to make inferences on species groups as they consist of multiple species that may vary in host preference and distribution. In the present study, R. neumanni was the most abundant species, but only present at 1 locality (60% prevalence at Kuruman). The Kuruman region is xeric (<340 mm per annum), which is in agreement with the species' observed preference for xeric regions in South Africa (Horak et al., Reference Horak, Heyne, Williams, Gallivan, Spickett, Bezuidenhout and Estrada-Peña2018). Micaelamys namaquensis is a new host record for R. neumanni that was previously reported on A. chrysophilus (1 larva) and Mastomys sp. (1 nymph) in Namibia (Horak et al., Reference Horak, Heyne, Williams, Gallivan, Spickett, Bezuidenhout and Estrada-Peña2018). In the present study, Rhipicephalus lunulatus was recorded at a single locality (Marken) and on <40% of the rodents. This tick species has been recorded previously on Elephantulus myurus (eastern rock sengi), A. chrysophilus and M. namaquensis in South Africa, but in low abundance (1–3 individuals per host species) (Horak et al., Reference Horak, Heyne, Williams, Gallivan, Spickett, Bezuidenhout and Estrada-Peña2018). Rhipicephalus follis was recorded at 4 localities of which 3 fall within the Grassland biome and the fourth (Hammanskraal) in the Savanna. The latter locality presents a new host and biome record for R. follis (Horak et al., Reference Horak, Heyne, Williams, Gallivan, Spickett, Bezuidenhout and Estrada-Peña2018). The low prevalence and abundance of Rhipicephalus distinctus in the present study (only recorded at Bethulie) is puzzling as this tick seems to have a preference for hosts that prefer rocky habitats such as P. capensis, E. myurus, Elephantulus edwardii (Cape rock elephant-shrew) and M. namaquensis (Horak et al., Reference Horak, Heyne, Williams, Gallivan, Spickett, Bezuidenhout and Estrada-Peña2018). Furthermore, R. distinctus was the most prevalent (67.1%) and abundant tick on M. namaquensis in the study by Fagir et al. (Reference Fagir, Ueckermann, Horak, Bennett and Lutermann2014). Several of the localities sampled during winter (June and July) are close to previous sampling records for the species (Horak et al., Reference Horak, Heyne, Williams, Gallivan, Spickett, Bezuidenhout and Estrada-Peña2018). It is possible that season played a role, as the larval stage appears to be more common during spring and summer (Horak et al., Reference Horak, Heyne, Williams, Gallivan, Spickett, Bezuidenhout and Estrada-Peña2018). Rhipicephalus exophthalmos was recorded at 2 localities (Postmasburg and Steynsburg), with 30% of the rodents at Postmasburg infested. Postmasburg is a new locality record for R. exophthalmos, which occurs throughout Namibia and has a patchy distribution in the south-western and southern parts of South Africa (Horak et al., Reference Horak, Heyne, Williams, Gallivan, Spickett, Bezuidenhout and Estrada-Peña2018). In addition, M. namaquensis is a new host recorded as the immature stages seem to have a preference for Macroscelides proboscideus (round-eared elephant-shrew), E. edwardii and Lepus saxatilis (scrub hare) (Horak et al., Reference Horak, Fourie, Novellie and Williams1991; Fourie et al., Reference Fourie, Horak and Woodall2005). Rhipicephalus warburtoni was abundant at 3 localities (Alldays 1, Hammanskraal and Steynsburg) that fall within the known distribution range of the species in South Africa. This tick is commonly associated with rocky outcrops and hosts that frequent these habitats. Rhipicephalus warburtoni has previously been recorded on M. namaquensis (Fagir et al., Reference Fagir, Ueckermann, Horak, Bennett and Lutermann2014), although it seems to prefer hares and E. myurus as hosts (Fourie et al., Reference Fourie, Horak and Woodall2005; Harrison et al., Reference Harrison, Robb, Bennet and Horak2012; Fagir et al., Reference Fagir, Horak, Ueckermann, Bennett and Lutermann2015). Hyalomma truncatum has a country-wide distribution and displays low host-specificity, with adults being generalists while immature stages are present on a range of small and medium-sized mammals such as Lepus capensis (Cape hares), L. saxatilis and murid rodents (Horak et al., Reference Horak, Fourie, Novellie and Williams1991, Reference Horak, Spickett, Braack and Penzhorn1993, Reference Horak, Spickett, Braack, Penzhorn, Bagnall and Uys1995, Reference Horak, Heyne, Williams, Gallivan, Spickett, Bezuidenhout and Estrada-Peña2018; Matthee et al., Reference Matthee, Horak, Beaucournu, Durden, Ueckermann and McGeoch2007). Studies on R. pumilio have recorded H. truncatum mainly from drier areas (e.g. Oudtshoorn) (S. Matthee, unpublished data). A similar pattern was recorded in the present study, with the species only recorded from localities with a mean annual rainfall <349 mm (Kuruman, Kimberley and Loeriesfontein). Haemaphysalis elliptica has been recorded throughout South Africa's Savanna, Grassland, Thicket, Fynbos and Succulent Karoo biomes (Horak et al., Reference Horak, Heyne, Williams, Gallivan, Spickett, Bezuidenhout and Estrada-Peña2018). Adult life stages prefer carnivores while the immature stages seem to prefer rodent hosts, particularly R. pumilio and M. namquensis but are also present on other small mammals (Fourie et al., Reference Fourie, Horak and van den Heever1992; Petney et al., Reference Petney, Horak, Howell and Meyer2004; Matthee et al., Reference Matthee, Horak, Beaucournu, Durden, Ueckermann and McGeoch2007). Nuttalliella cf. namaqua was represented by a single individual (larva) recorded from a single rodent at Loeriesfontein in the Northern Cape. It is possible that this tick is N. namaqua, a monotypic species within the family Nuttalliellidae. This is based on the fact that M. namaquensis is regarded as a preferred host for N. namaqua and the latter species has been recorded in regions close and with similar climatic conditions to Loeriesfontein (Mans et al., Reference Mans, de Klerk, Pienaar and Latif2011; Horak et al., Reference Horak, Lutermann, Medger, Apanaskevich and Matthee2012; Apanaskevich, Reference Apanaskevich2021). In addition to the immature stages, 2 adult female I. bakeri-like ticks were collected. Although species in the genus Ixodes usually displays a strong on-host female bias (Horak et al., Reference Horak, Heyne, Williams, Gallivan, Spickett, Bezuidenhout and Estrada-Peña2018), very few adult ticks were collected to confirm this in the present study. Several of the tick species that were recorded in the study are known vectors for disease-causing pathogens or are directly responsible for causing disease. In particular, H. elliptica is a vector of Babesia rossi, the causative agent for canine babesiosis in South Africa (Matjila et al., Reference Matjila, Penzhorn, Bekker, Nijhof and Jongejan2004, Reference Matjila, Leisewitz, Jongejan and Penzhorn2008). Hyalomma truncatum is known to transmit Babesia caballi to horses (de Waal, Reference de Waal1990), and females of certain strains of H. truncatum may secrete toxins in their saliva, which may cause sweating sickness in cattle, with greater risk for calves (Neitz, Reference Neitz1956). Hyalomma truncatum is also a vector of Crimean-Congo haemorrhagic fever virus to humans (Hoogstraal, Reference Hoogstraal1979; Swanepoel et al., Reference Swanepoel, Shepherd, Leman, McGillivray, Erasmus, Searle and Gill1987). In Zimbabwe, R. lunulatus has been associated with tick paralysis in calves and lambs. The adults of R. neumanni occur between the claws of sheep and may cause lameness and the formation of abscesses (Walker, Reference Walker1990). Lastly, R. warburtoni has been reported as causing paralysis in new-born Angora goats (Horak et al., Reference Horak, Heyne, Williams, Gallivan, Spickett, Bezuidenhout and Estrada-Peña2018).
The current study in addition to published literature highlights the rich diversity of ectoparasite taxa that are associated with the opportunistic and regionally widespread M. namaquensis (Table 7). Several of the tick and flea species are of medical and/or veterinary importance and it is therefore important to monitor the population sizes of M. namaquensis in anthropogenic habitats. Further, the study makes novel contributions in terms of new locality and host records for several ectoparasite and undescribed chigger species. Data on the chigger diversity and mite and louse geographic distribution provide valuable baseline information for future studies on rodent ectoparasites.
Table 7. Summary of ectoparasite taxa associated with Micaelamys namaquensis (previously Aethomys namaquensis) to date

a Species of veterinary and medical importance.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182022000750.
Data availability
All data generated or analysed during this study are included in this published article. The datasets used and/or analysed are available from the corresponding author upon reasonable request.
Acknowledgements
We thank the various landowners and conservation bodies that allowed sampling on their properties or in the reserves under their management. JC Bothma, Conrad and Johann Matthee, Nico Avenant, Lourens Swanepoel and Dylan Smith assisted in the field and contributed samples. Several research assistants and Luther van der Mescht supported the laboratory work. Lance Durden provided guidance with regard to the Hoplopleura patersoni–Hoplopleura aethomydis lice query.
Author contributions
S. M. conceived the study and supervised L. S. L. S. conducted the laboratory work and wrote the draft chapters of the manuscript. A. A. S. and I. G. H. assisted with the identification of chiggers and ticks, respectively. E. A. U. identified the mites. All authors contributed to the final version of the manuscript.
Financial support
This research project was supported by Stellenbosch University, The National Research Foundation (NRF)-Incentive Funding (grant number 85718 to S. M.) and a NRF-FBIP bursary (grant number 128323 to L. S.). Any opinion, finding and conclusion or recommendation expressed in this material is that of the author(s) and the NRF does not accept any liability in this regard. The identification of chiggers was supported by the Ministry of Science and Higher Education of the Russian Federation (grant number 122031100263-1 to A. A. S).
Conflict of interest
None.
Ethical standards
The ectoparasite material used in the study was obtained from a previous study conducted on the molecular ecology of sucking lice (Anoplura) associated with the Aethomys/Micaelamys rodent complex (Bothma et al., Reference Bothma, Matthee and Matthee2020, Reference Bothma, Matthee and Matthee2021). The project was approved by Stellenbosch Animal Ethics (SU-ACU-2018-4555) and permits obtained (Limpopo, ZA/LP/90994; North-West, NW 7705; Eastern Cape, CRO 150/17CR and CRO 11/17CR; Northern Cape, FAUNA 0942/2017 and FAUNA 0949/2017).