Introduction
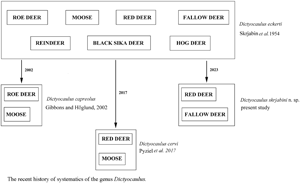
Lungworms of the genus Dictyocaulus Railliet and Henry, Reference Railliet and Henry1907 (Nematoda: Trichostrongyloidea) are causative agents of parasitic bronchitis in domestic and wild ungulates (Hӧglund et al., Reference Höglund, Morrison, Divina, Wilhelmsson and Mattson2003; Ács et al., Reference Ács, Hayward and Sugár2016; Pyziel et al., Reference Pyziel, Dolka, Werszko, Laskowski, Steiner-Bogdaszewska, Wiśniewski, Demiaszkiewicz and Anusz2018a). In the first systematic revision of the genus, D. eckerti Skrjabin, 1931 was described from a reindeer (Skrjabin et al., Reference Skrjabin, Shikhobalova, Schultz, Skrjabin, Shikhobalova and Schultz1954). The most current systematic revision of the genus maintained D. eckerti as a collective species for all cervids (Gibbons and Khalil, Reference Gibbons and Khalil1988). Following the retrospective description of D. capreolus Gibbons and Höglund, Reference Gibbons and Höglund2002 from roe deer and moose, and D. cervi Pyziel, Laskowski, Demiaszkiewicz and Höglund, Reference Pyziel, Laskowski, Demiaszkiewicz and Höglund2017 from red deer, the classification was revised, and the species were separated from D. eckerti (Gibbons and Hӧglund, Reference Gibbons and Höglund2002; Pyziel et al., Reference Pyziel, Laskowski, Demiaszkiewicz and Höglund2017).
Dictyocaulus cervi was described on the basis of the unique ribosomal SSU, ITS2 and mitochondrial cox1 sequences, as well as on the morphological characteristics of male and female lungworms obtained from red deer inhabiting north-eastern Poland. Interestingly, D. cervi was also detected in wild red deer in 2 areas in the Italian Alps (Cafiso et al., Reference Cafiso, Castelli, Tedesco, Poglayen, Buccheri Pederzoli, Roretto, Orusa, Corlatti, Bazzocchi and Luzzago2023), as well as in a rocky mountain elk Cervus canadensis nelsoni in the USA (Bangoura et al., Reference Bangoura, Brinegar and Creekmore2021). Moreover, some nucleotide sequences derived from lungworms of red deer from Hungary were found to match sequences of D. cervi (Ács et al., Reference Ács, Hayward and Sugár2016). Thus, the species seems to be present not only in Eastern Europe but also in various other locations worldwide. Infection with D. cervi has been associated with various manifestations of lung pathology, including interstitial pneumonia, bronchitis and bronchiolitis with an influx of eosinophils, lymphocytes, plasma cells and macrophages; massive hyperplasia of lymphoid follicles in bronchiolar tissue and hyperplasia of bronchial and bronchiolar epithelium (Pyziel et al., Reference Pyziel, Dolka, Werszko, Laskowski, Steiner-Bogdaszewska, Wiśniewski, Demiaszkiewicz and Anusz2018a).
D. cervi differs from D. eckerti regarding the absence of cervical papillae, the presence of a single ring of 4 symmetrical submedian cephalic papillae, the length of the tail in females, the morphometry of the female reproductive organs and the dimensions of the gubernacula in males (Pyziel et al., Reference Pyziel, Laskowski, Demiaszkiewicz and Höglund2017). Nevertheless, it should be kept in mind that in wild cervids, there is a higher risk of misidentification of Dictyocaulus when the identification is based solely on morphology (Divina et al., Reference Divina, Wilhelmsson, Mattsson, Waller and Höglund2000). Therefore, it is recommended to complement the morphological examination with genetic analysis to enable accurate identification (Nadler and Pérez-Ponce de León, Reference Nadler and Pérez-Ponce de León2011).
Taxonomic differentiation of Dictyocaulus spp. can be facilitated by using the small subunit (SSU) and ITS2 ribosomal rDNA as genetic markers (Epe et al., Reference Epe, Samson-Himmelstjerna and Schnider1997, Hӧglund et al., Reference Höglund, Wilhelmsson, Christensson, Mörner, Waller and Mattsson1999; Pyziel, Reference Pyziel2014), as well as more variable mitochondrial (mt) DNA markers (Hӧglund et al., Reference Höglund, Morrison, Mattsson and Engström2006; Gasser et al., Reference Gasser, Jabbar, Mohandas, Höglund, Hall, Littlewood and Jex2012). Our previous studies suggest that SSU of ribosomal rDNA and cytochrome B (cytB) of mt DNA are well conserved, and they seem to be the most suitable markers for systematic and molecular epidemiological studies of Dictyocaulus spp. (Pyziel et al., Reference Pyziel, Laskowski, Dolka, Kołodziej-Sobocińska, Nowakowska, Klich, Bielecki, Żygowska, Moazzami, Anusz and Höglund2020). The sequences of other mt markers are more variable within lungworm species, and are therefore more suitable for studying population genetics (Hu et al., Reference Hu, Höglund, Chilton, Zhu and Gasser2002; Hӧglund et al., Reference Höglund, Morrison, Mattsson and Engström2006; Gasser et al., Reference Gasser, Jabbar, Mohandas, Höglund, Hall, Littlewood and Jex2012; Ács et al., Reference Ács, Hayward and Sugár2016; Pyziel et al., Reference Pyziel, Dolka, Werszko, Laskowski, Steiner-Bogdaszewska, Wiśniewski, Demiaszkiewicz and Anusz2018a).
Although knowledge about the species composition and genetic variability of Dictyocaulus spp. continues to increase, studies still report the emergence of new, enigmatic genotypes of lungworms in wild ruminants (Ács et al., Reference Ács, Hayward and Sugár2016; Cafiso et al., Reference Cafiso, Castelli, Tedesco, Poglayen, Buccheri Pederzoli, Roretto, Orusa, Corlatti, Bazzocchi and Luzzago2023). It appears that the species composition and genetic variability within the genus Dictyocaulus continues to expand (Bangoura et al., Reference Bangoura, Brinegar and Creekmore2021; Danks et al., Reference Danks, Sobotyk, Saleh, Kulpa, Luksovsky, Jones and Verocai2022); for example, a lungworm with different ITS2 sequence data has been discovered in fallow deer in Sweden (Divina et al., Reference Divina, Wilhelmsson, Mӧrner, Mattsson and Höglund2002). The present study investigates the distribution, morphology and genetic diversity of Dictyocaulus spp. in red deer in Poland and in red deer, fallow deer and moose in Sweden.
Materials and methods
Specimen collection
The study was conducted on 167 individuals of red deer culled during 2017/2018 and 2018/2019 hunting seasons in Poland as part of a management strategy. Lungs, together with trachea, were collected from 65 animals from the Lower Silesian Wilderness (51° N 15° E, south-western Poland), 83 from the Bieszczady Mountains (49° N 22° E, south-eastern Poland), and 19 as control material from the Piska Forest (53° N 22° E, north-eastern Poland): an area where D. cervi was previously recorded as the only Dictyocalus species infecting red deer (Pyziel et al., Reference Pyziel, Laskowski, Demiaszkiewicz and Höglund2017). Briefly, all red deer were dissected and their trachea, bronchi and bronchioles were cut open. Any adult Dictyocaulus sp. specimens were collected in laboratory tubes containing 70% ethanol and taken for testing.
DNA extraction, amplification and sequencing
Depending on the intensity of infection, 1 to 5 male lungworms from each respiratory tract of the red deer examined were subjected to molecular analysis. Genomic DNA was extracted individually from 80 adult male lungworms using a Nucleospin tissue DNA extraction kit (Macherey-Nagel, Düren, Germany) according to the manufacturer's protocol.
In addition, DNA samples of lungworms were derived from 7 fallow deer, 4 red deer and 2 moose from Sweden collected for a previous study (Höglund et al., Reference Höglund, Morrison, Divina, Wilhelmsson and Mattson2003). Regions of the small subunit ribosomal RNA (SSU), and mitochondrial cytochrome B (cytB) of the worms were amplified by PCR using the following primers sets : NF50 (5′-TGA AAC TGC GAA CGG CTC AT-3′) + BNR1 (5'-ACC TAC AGA TAC CTT GTT ACG AC-3') for SSU (Pyziel et al., Reference Pyziel, Laskowski, Demiaszkiewicz and Höglund2017); cytB_F (5'-TGA AAA RGT TAA GAT RRT TGG GAC-3') + cytB_R (5'-TTA GGA ATA GCA CGC AAA ATA GC-3') for cytB (Pyziel et al., Reference Pyziel, Laskowski, Dolka, Kołodziej-Sobocińska, Nowakowska, Klich, Bielecki, Żygowska, Moazzami, Anusz and Höglund2020). Genetic markers were selected based on previous studies (Pyziel et al., Reference Pyziel, Laskowski and Höglund2018b, Reference Pyziel, Laskowski, Dolka, Kołodziej-Sobocińska, Nowakowska, Klich, Bielecki, Żygowska, Moazzami, Anusz and Höglund2020). Primers were designed using FastPCR software, version 5.4 (Primer Digital, Helsinki, Finland).
PCR was performed in a 2720 thermal cycler (Applied Biosystems, Foster City, California) in a 50 μl volume. Each 50 μl PCR reaction contained 20 μl of Molecular Biology Reagent Water (Sigma-Aldrich, USA), 25 μl Quant-Bio's AccuStartTM II PCR ToughMix® ( × 2 concentration) (Quantabio, Beverly, USA), 1 μl GelTrack Loading Dye (×50 concentration) (Quantabio, Beverly, USA), 1 μl forward primer (20 mm), 1 μl reverse primer (20 mm) and 2 μl template DNA.
The conditions for PCR were as follows: 94°C for 2 min to denature DNA, with 35 cycles at 94°C for 40 s (SSU)/45 s (cytB), 55°C for 1.5 min (SSU)/56°C for 60 s (cytB), and 72°C for 2 min (SSU)/45 s (cytB); with a final extension of 10 min at 72°C to ensure complete amplifications.
The PCR product was purified with the Nucleospin Gel and PCR Clean-up Kit (Macherey-Nagel, Germany), eluted with 30 μl Molecular Biology Reagent Water (Sigma-Aldrich, USA) and sequenced in both directions by Macrogen Europe (Amsterdam, the Netherlands) using the primers (5 mm) used for amplification. The sequences were then assembled into contigs using CodonCode Aligner version 8.0 (CodonCode Corporation, Massachusetts, USA).
Phylogenetic analysis
Phylogenetic analysis of the taxa of Dictyocaulus was performed by alignment with the partial nucleotide sequences of SSU (1,605 bp) and the amino acid sequences of the partial nucleotide sequences of cytB (642 bp), and using the GTR + I (cytB) and GTR + G (SSU) evolutionary model. A list of taxa included in the molecular analyses is listed in Table S1 (SSU), Table S2 (cytB). Evolutionary models were selected based on JModelTest 2.1.10 (Guindon and Gascuel, Reference Guindon and Gascuel2003; Darriba et al., Reference Darriba, Taboada, Doallo and Posada2012) using the AIC criterion. The phylogenetic tree was constructed using Bayesian inference analysis (BI) with MrBayes version 3.2. (Huelsenbeck and Ronquist, Reference Huelsenbeck and Ronquist2001).
Morphological characteristics
Molecular preselection led to the identification of a new lungworm genotype in 12 of the 64 Dictyocaulus-positive red deer: 4 individuals from the Lower Silesian Wilderness and 8 from the Bieszczady Mountains. These animals served as source for 54 nematodes (19 males and 35 females) which were examined morphologically. The worms were cleared in lactophenol and mounted on slides in glycerine jelly. Measurements and microphotographs were made using an Opta-Tech Lab40 light microscope (×40 –×1000 magnification) and the OptaView IS-PL Opta-Tech software package (Opta-Tech, Warsaw, Poland).
The following measurements were taken: body length, buccal capsule (BC), buccal capsule wall (BCW), head, cephalic vesicle and oesophagus of males and females; copulatory bursa, gubernaculum, spicules of the male reproductive system; vestibules, anterior sphincter and infundibulum, posterior sphincter and infundibulum, tail and phasmids of the female reproductive system. The location of the nerve ring, excretory pore and opening of the vulva were also examined.
Specimens destined for scanning electron microscopy (s.e.m.) were dehydrated in increasing concentrations of ethanol and stored in acetone (Eisenback, Reference Eisenback, Barker, Carter and Sasser1985). The dehydrated specimens were then dried at the critical-point with liquid CO2; their proximal endings were then cut and mounted on a s.e.m. mounting stub with double-coated adhesive tape, sputter-coated with gold, and examined with a LEO 1430VP (ZEISS, Jena, Germany) scanning electron microscope.
Statistical analysis
The range, mean and standard deviation of the morphological measurements of D. skrjabini n. sp. were compared with those of D. cervi presented by Pyziel AM et al. (Reference Pyziel, Laskowski, Demiaszkiewicz and Höglund2017). The normality of each variable was first checked with the Shapiro–Wilk test.
The following morphological traits showed a normal distribution: length of the body, cephalic vesicle, BCW, anterior to nerve ring, anterior to excretory pore, copulatory bursa, gubernaculum, spicules, posterior to vulva opening, vestibules, anterior sphincter, anterior infundibulum, posterior sphincter, posterior infundibulum, posterior to anus (tail), posterior to phasmids; the width of BC, BCW, oesophagus max., body at vulva opening. These were compared using the Student's T test.
Other morphological traits, namely the length of the oesophagus and BC, and the width of the head and cephalic vesicle, were compared with the Mann–Whitney U-test. Probability values less than 0.05 (P < 0.05) were considered statistically significant.
Results
Prevalence and intensity of infection
Dictyocaulus spp. lungworms were diagnosed in 27 of 65 respiratory tracts of red deer from the Lower Silesian Wilderness (prevalence = 41.5%), in 26 of 83 from the Bieszczady Mountains (prevalence = 31.3%), and in 11 of 19 from the Piska Forest (prevalence = 57.9%). The mean intensity of infection was 13.7 ± 19.9 in the Lower Silesian Wilderness (range = 1–78), 11.1 ± 20.6 in the Bieszczady Mountains (range = 1–98), and 12.6 ± 10.5 from the Piska Forest (range = 1–39).
Both the red deer populations from the Lower Silesian Wilderness and from the Bieszczady Mountains were infected with D. cervi and a new species of large lungworm (Dictyocaulus skrjabini n. sp.); however, the red deer from the Piska Forest harboured D. cervi exclusively. None of the animals from the Lower Silesian Wilderness or the Bieszczady Mountains was found to have mixed infection with the 2 species: each Dictyocaulus spp. – positive red deer was infected with only one species. In both areas, D. cervi was overwhelmingly more prevalent in red deer than D. skrjabini n. sp.
Dictyocaulus skrjabini n. sp. was found in the respiratory tract of 4 individuals from the Lower Silesian Wilderness (prevalence = 6.1%), and in 8 from the Bieszczady Mountains (prevalence = 9.6%). The mean intensity of infection with D. skrjabini n. sp. was 7.5 ± 4.5 in the Lower Silesian Wilderness (range = 4–14), and 19.5 ± 32.8 in the Bieszczady Mountains (range = 1–98). The remaining Dictyocaulus belonged to D. cervi species.
DNA sequences
The study revealed 186 new nucleotide sequences of the marker genes SSU and mt cytB. Thirteen slightly different sequences derived from different hosts were submitted to GenBank [MN448405 – MN448408, MH756628, MH756629 (SSU); MN503296 – MN503299, MN503302 – MN503304 (cytB)]. The SSU sequences varied in length from 1,639 base pairs (bp) to 1,652 bp, and cytB from 693 bp to 744 bp.
In Sweden, all 7 isolates of fallow deer lungworms (GenBank: MN448406, MN503299, MN450300) and one isolate of lungworms derived from red deer (GenBank: MN503303) contained D. skrjabini n. sp. In addition, 3 isolates of lungworm from red deer (GenBank: MN503302, MN448405) and 2 from moose, i.e. from both studied individuals (GenBank: MN448407), were found to be D. cervi.
For the 1,605 bp SSU sequence, D. skrjabini n. sp. differed by 14 nucleotides from D. cervi and by 15 nucleotides from D. eckerti (0.9% sequence divergence) (Table 1).
Table 1. Pairwise comparison of small subunit rDNA sequence variability among 6 species (9 selected isolates) of Dictyocaulus

Number of variable sites in 1,605 base pair. Percentage of variable sites between 2 species/isolates is given in parentheses.
1: AJ920362, 2: MH756629, 3: AJ920361, 4: KC771250, 5: AY168859, 6:MG833326, 7: MG833324, 8: MT919232, 9: AY168864.
As for the 642 bp cytB sequence, the nucleotide sequence variation within the species ranged from 2 to 6 nucleotides depending on the D. skrjabini n. sp. isolate (0.3–0.9% of sequence divergence). However, no variability was observed in the 214 amino acid sequence (Table 2). The cytB gene sequence differed by 106–111 nucleotides between D. skrjabini n. sp. and D. cervi (16.5–17.3% nucleotide sequence divergence).
Table 2. Pairwise comparison of cytochrome b mitochondrial DNA nucleotide sequence and inferred amino acid sequence variability among 4 species (14 selected isolates) of Dictyocaulus

Above diagonal = number of variable sites in 642 base pairs. Below diagonal = number of variable sites of 214 amino acids. Percentage of variable sites between 2 species/isolates is given in parentheses.
1: MN503304, 2: MN503296; 3: MN503302, 4: MT920216, 5: MT920217, 6: MT920218, 7: JX519459, 8: MN503303, 9: MN503297, 10: MN503299, 11: MN503298, 11: JX519460, 12: AP017683, 13: MN503300; 14: MN503301.
When the nucleotide sequences were translated into amino acid sequences, they differed by 39–40 amino acids (18.2–18.7% of amino acid sequence divergence). The nucleotide sequence divergence in the cytB gene between D. skrjabini n. sp. and D. eckerti ranged from 120 to 122 nucleotides (i.e. 18.7–19% divergence); this corresponds to a difference of 40 amino acids between them (i.e. 18.7% divergence) (Table 2).
Phylogenetic reconstruction
Bayesian analysis (BI) of the SSU rDNA sequence data, with Dictyocaulus filaria as an outgroup, revealed 3 strongly supported clades (Fig. 1). One clade containing D. filaria, the second clade containing D. skrjabini n. sp. taxa from red deer and fallow deer, and the third clade containing 3 subclades: one subclade containing D. viviparus from cattle and European bison, the other subclade containing D. capreolus and Dictyocaulus sp. isolates from roe deer, the third subclade containing D. cervi from red deer and moose. In the third subclade, D. eckerti was a sister taxon of D. cervi.

Figure 1. Phylogenetic tree of Dictyocaulus spp. based on SSU rDNA partial sequences, constructed with the use of Bayesian inference (BI) analysis using MrBayes version 3.2. The GTR + G model was chosen based on jModelTest version 2.1.4 using Akaike information criterion. The analysis was run for 1 000 000 generations, with 500 000 generations discarded as ‘burn-in’. GenBank accession numbers, hosts and country of origin are shown. Nodal support is indicated as Bayesian posterior probabilities. Sequence from Dictyocaulus filaria (AJ920362) was used as an outgroup.
In the BI analysis of cytB sequence data with Aelurostrongylus abstrusus as the outgroup, D. skrjabini n. sp. and A. abstrusus formed 2 independent clades (Fig. 2). The third cluster comprised 2 subclades: a subclade with D. viviparus from cattle and European bison, and a subclade with D. eckerti from red deer with D. cervi as a sister taxon. The D. cervi sequences were found to be heterogeneous.

Figure 2. Phylogenetic tree of Dictyocaulus spp. based on cytB partial sequences, constructed with the use of Bayesian inference (BI) analysis using MrBayes version 3.2. The GTR + I model was chosen based on jModelTest version 2.1.4 using Akaike information criterion. The analysis was run for 1 000 000 generations, with 500 000 generations discarded as ‘burn-in’. GenBank accession numbers, hosts and country of origin are shown. Nodal support is indicated as Bayesian posterior probabilities. Sequence from Aelurostrongylus abstrusus (JX519458) was used as an outgroup.
Description of Dictyocaulus skrjabini n. sp.
General morphology (based on 54 specimens: 19 males and 35 females): BC oval, dorsoventrally flattened (Figs 3A, B, D). Oral opening elongate oval, dorsoventrally flattened. Thickness of BCW (range = 42–96 μm) thick, according to Divina et al. (Reference Divina, Wilhelmsson, Mattsson, Waller and Höglund2000). Single ring of 4 symmetrical submedian cephalic papillae, lateral amphids absent (Fig. 3D). Cuticle with numerous longitudinal ridges. Cervical papillae absent, nerve ring (Fig. 3C) and excretory pore difficult to discern.

Figure 3. Dictyocaulus skrjabini n. sp. of red deer, anterior end. (A) Anterior end in optical section, showing head, cephalic vesicle, oesophagus, lateral view. (B) Anterior end in optical section, showing buccal capsule (bc), buccal capsule wall (bcw), cephalic vesicle, lateral view. (C) Anterior end below head region in optical section, showing nerve ring (nr), lateral view. (D) Cephalic region, scanning electron microscopy, showing BC and 4 submedian papillae (SCP).
Male (holotype): Body 8.2–46.6 mm long. Head 91–144 μm wide. Cephalic vesicle present, 126–207 μm long and 17–45 μm wide. Oesophagus 901–1,449 μm long, 117–297 μm maximum width. BC 14–39 μm wide, 40–53 μm long. BCW 5–9 μm wide and 52–96 μm long. Anterior to nerve ring 348–491 μm. Copulatory bursa 133–523 μm long (Fig. 4A). Spicules 195–306 μm long, porous texture, dark brown, small easily visible transparent membrane around the tip of each spicule (Fig. 4B). Gubernaculum present, 46–71 μm long, 25–36 μm width, porous texture, paler than spicules, irregularly oval in dorsoventral view, longitudinal, slightly curved at distal end, which is thinner than proximal end in lateral view (Figs 4A, B, D). Bursa bell shaped, lobes not separated, heart-shaped in dorsoventral view (Fig. 4C). Ventral rays parallel, with short common stem, anteroventral shorter than posteroventral, reaching about two-thirds of its length, not reaching bursal margin, posteroventral almost reaching bursal margin; anterolateral ray separate, short, not reaching bursal margin, with rounded distal tip; mediolateral and posterolateral rays completely fused, long, almost reaches bursal margin (Fig. 4C); externodorsal ray separate from, and shorter than, dorsal ray, with rounded distal tip; dorsal ray divided at base, each branch with 3 small divisions at distal tip, almost reaches bursal margin.
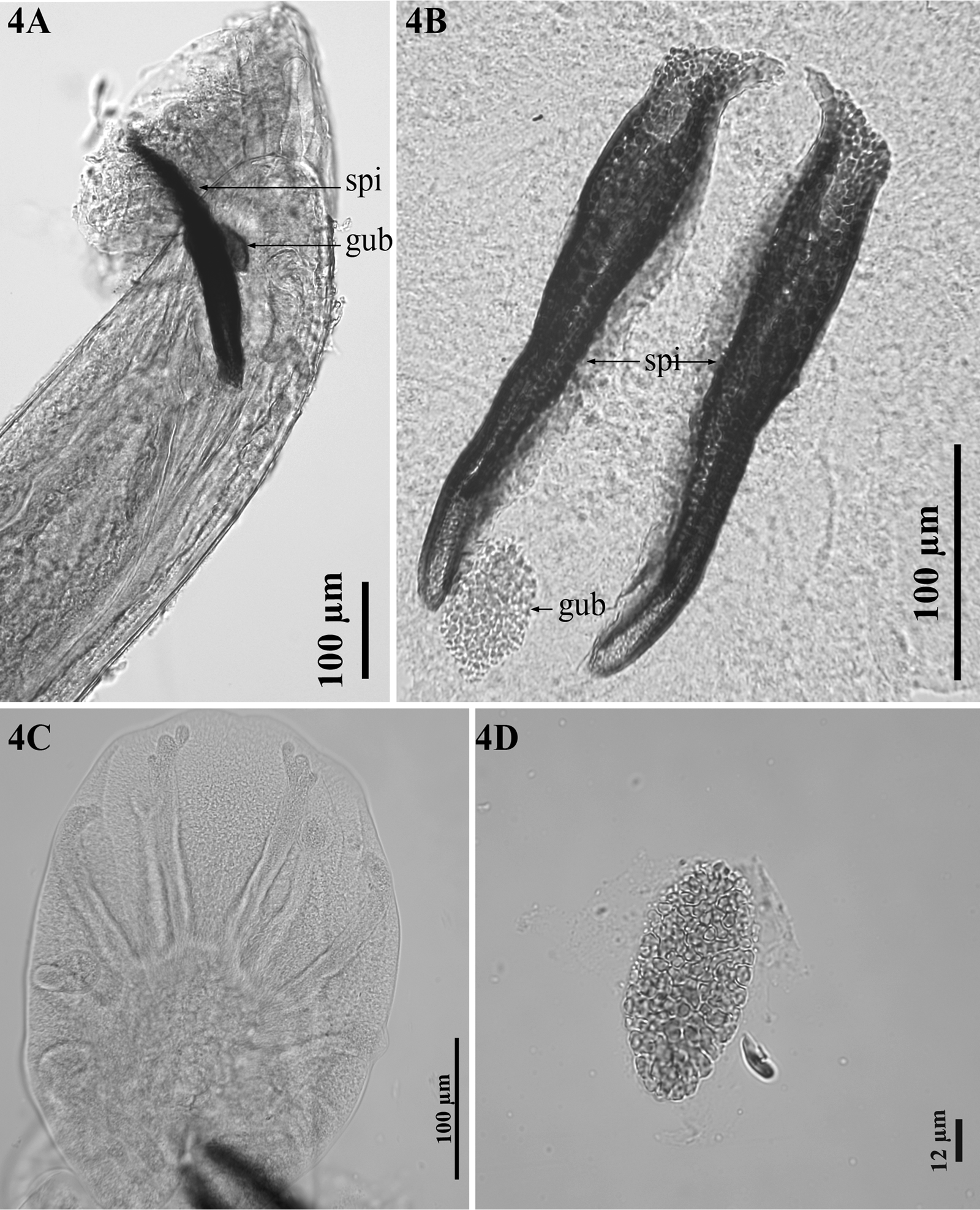
Figure 4. Dictyocaulus skrjabini n. sp. of red deer, male genital system, light microscopy. (A) Bursa, showing left spicula (spi) and gubernaculum (gub), lateral view. (B) Spicules (spi), gubernaculum (gub), dorsal view. (C) Bursa, abdominal view. (D) Gubernaculum, dorsal view.
Female (allotype): Body 20.1–60.7 mm long. Head 96–183 μm wide. Cephalic vesicle present, 162–219 μm long and 18–35 μm wide. Oesophagus 893–1,514 μm long and 121–231 μm maximum width. BC 16–49 μm wide, 36–81 μm long. BCW 4–10 μm wide and 42–96 μm long. Anterior to nerve ring 356–463 μm. Vulva opening 18–25.2 mm from posterior end, vulval lips are slightly swollen (Fig. 5A). Body width at vulval opening 421–661 μm. Reproductive apparatus didelphic, amphidelphic (Fig. 5A). Combined length of opposed vestibules 1,189–2,523 μm. Length of anterior sphincter 58–167 μm and length of anterior infundibulum 23–62 μm (Fig. 5B). Length of posterior sphincter 48–114 μm and length of posterior infundibulum 37–74 μm (Fig. 5C). Tail 313–483 μm long; phasmids 132–210 μm from posterior end (Fig. 5D).

Figure 5. Dictyocaulus skrjabini n. sp. of red deer, female genital system, light microscopy. (A) Ovejectors in right lateral view, showing relationships for the vulva (vu), vestibules and combined anterior infundibulum and sphincter (ainf + asph) and posterior infundibulum and sphincter (pinf + psph). (B) Region of anterior infundibulum (ainf) and anterior sphincter (asph), right lateral view. (C) Region of posterior infundibulum (pinf) and posterior sphincter (psph), right lateral view. (D) Female tail, right lateral view, showing anus and phasmids (ph).
Taxonomic summary
Type host: red deer, Cervus elaphus (Linnaeus, 1758) (Artiodactyla: Cervidae).
Other known host: fallow deer, Dama dama (Linnaeus, 1758) (Artiodactyla: Cervidae).
Site of infection: trachea, bronchi, bronchioles.
Type locality: Bieszczady Mountains, Poland (49° N 22° E).
Distribution: Lower Silesian Wilderness, Poland (51° N 15° E), Sweden (lack of detailed information).
Deposited specimens: Museum of Natural History, University of Wroclaw, Poland; holotype (male) (from Bieszczady Mountains, Poland) No. MNHW 1436a, and allotype (female) (from Lower Silesian Wilderness, Poland) No. MNHW 1436b.
Prevalence of infection: In 12 of 167 (7.2%).
Deposited sequences: GenBank MH756629 [SSU rDNA; Cervus elaphus; Bieszczady Mountains, Poland (49° N 22° E)], MN448408 [SSU rDNA; Cervus elaphus; Lower Silesian Wilderness, Poland (51° N 15° E)], MN472750 [ITS2 rDNA; Cervus elaphus; Bieszczady Mountains, Poland (49° N 22° E)], MN503298 [mt-cytB; Cervus elaphus; Bieszczady Mountains, Poland (49° N 22° E)], MN503297 [mt-cytB; Cervus elaphus; Lower Silesian Wilderness, Poland (51° N 15° E)], MN448406 (SSU rDNA; Dama dama; Sweden), MN503299 (mt-cytB; Dama dama; Sweden), MN450300 (ITS2 rDNA; Dama dama; Sweden), MN503303 (mt-cytB; Cervus elaphus; Sweden).
Etymology: The specific epithet derives from the name of professor Konstantin Ivanovich Skrjabin, a veterinary parasitologist whose study led to the description of Dictyocaulus eckerti from reindeer, Rangifer tarandus. Since then D. eckerti was maintained as a collective species infecting various cervid hosts. This arrangement was actual until D. capreolus and D. cervi were described and segregated from D. eckerti.
Remarks
According to Divina et al. (Reference Divina, Wilhelmsson, Mattsson, Waller and Höglund2000), Dictyocaulus lungworms can be distinguished from each other morphologically mainly by BCW thickness and length. However, there are currently no available data on the BCW of D. eckerti, which makes any comparison with other Dictyocaulus species impossible (Pyziel et al., Reference Pyziel, Laskowski, Demiaszkiewicz and Höglund2017). In the present study, the BCW of D. skrjabini n. sp. was statistically significantly longer and narrower than that of D. cervi. The mean length and width of the BCW of D. skrjabini was 63.4 ± 12.5 and 6.7 ± 1.1 in males, whereas it was 65 ± 10.3 and 7.3 ± 1.4 in females, respectively (Tables S3 and S5). The mean length and width of the BCW of D. cervi was 19.5 ± 6.4 and 7.8 ± 1.7 in males, whereas it was 23.9 ± 5.4 and 8.2 ± 1.5 in females, respectively (Pyziel et al., Reference Pyziel, Laskowski, Demiaszkiewicz and Höglund2017). Moreover, according to Divina et al. (Reference Divina, Wilhelmsson, Mattsson, Waller and Höglund2000), the BCW was described as thick in D. skrjabini n. sp. and as medium in D. cervi. Although, a single ring of 4 symmetrical submedian cephalic papillae was observed in both D. skrjabini n. sp. and D. cervi, no lateral amphids were seen in D. skrjabini n. sp. compared to D. cervi, which bore 2 lateral amphids at its anterior extremity. In contrast, 2 rings of size-differentiated cephalic papillae were observed in D. eckerti consisting of 4 papillae in the external ring and 6 smaller papillae in the internal ring (Skrjabin et al., Reference Skrjabin, Shikhobalova, Schultz, Skrjabin, Shikhobalova and Schultz1954). Furthermore, in contrast to D. eckerti, no cervical papillae were observed in either D. skrjabini n. sp. or D. cervi, (Skrjabin et al., Reference Skrjabin, Shikhobalova, Schultz, Skrjabin, Shikhobalova and Schultz1954; Gibbons and Khalil, Reference Gibbons and Khalil1988). The nerve ring was visible in both D. skrjabini n. sp. and D. cervi, but not observed in D. eckerti (Gibbons and Khalil, Reference Gibbons and Khalil1988). Furthermore, no statistically significant differences in the length of the spicules were observed between D. skrjabini n. sp. and D. cervi, which is consistent with previous observations that the length of the spicules is not a reliable morphological trait for Dictyocaulus spp. (Gibbons and Khalil, Reference Gibbons and Khalil1988; Divina et al., Reference Divina, Wilhelmsson, Mattsson, Waller and Höglund2000).
Females of D. skrjabini n. sp. were significantly shorter than females of D. cervi; however, no statistically significant difference in total body length was found between males of the 2 species (Table S3). Similarly, females of D. skrjabini n. sp. were wider at the vulval opening compared to those of D. cervi (Table S4). Regardless of sex, individuals of D. skrjabini n. sp. were characterized by a longer cephalic vesicle, BC, BCW, and a greater distance from the anterior extremity to the nerve ring compared to individuals of D. cervi (Table S3). The distance from the anterior extremity to the excretory pore was longer in the males of D. skrjabini n. sp., but shorter in the females of D. skrjabini n. sp., compared to individuals of D. cervi (Table S3). In both males and females of D. skrjabini n. sp. the head and oesophagus were wider than in D. cervi (Table S5). In contrast, cephalic vesicle, BC and BCW were narrower in D. skrjabini n. sp. compared to D. cervi, regardless of sex (Table S5).
The only statistically significant difference in the male reproductive system concerned the length of the gubernaculum, which was shorter in D. skrjabini n. sp. than in D. cervi (Table S6). In the female reproductive system, both vestibules and the anterior infundibulum were shorter in D. skrjabini n. sp. than in D. cervi; while the anterior sphincter and posterior sphincter were longer in D. skrjabini n. sp. (Table S4).
Discussion
The genus Dictyocaulus was established by Railliet and Henry (Reference Railliet and Henry1907) for large lung nematodes recovered from respiratory tract of artiodactylids. It was originally considered to have 4 species, namely D. viviparus (specific to cattle), D. filaria (specific to sheep and goat), D. arnfieldi (specific to donkeys and horses) and D. noerneri (specific to cervids). According to the first systematic revision of the genus (Skrjabin et al., Reference Skrjabin, Shikhobalova, Schultz, Skrjabin, Shikhobalova and Schultz1954), D. cameli (specific to camels) was established and D. noerneri was replaced with D. eckerti, as D. noerneri was considered invalid. The second systematic revision of the genus Dictyocaulus (Gibbons and Khalil, Reference Gibbons and Khalil1988) resulted in description of D. africanus (specific to African artiodactylids). Since its erection, D. eckerti was maintained as a collective species infecting various cervid hosts including reindeer, red deer, elk, roe deer, moose, fallow deer, black sika deer, and hog deer (Skrjabin et al., Reference Skrjabin, Shikhobalova, Schultz, Skrjabin, Shikhobalova and Schultz1954; Romano and Persiani, Reference Romano and Persiani1982; Gibbons and Khalil, Reference Gibbons and Khalil1988; Jansen and Borgsteede, Reference Jansen and Borgsteede1990). This arrangement was actual until D. capreolus (Gibbons and Hӧglund, Reference Gibbons and Höglund2002) from roe deer and moose was described. Since the description of D. capreolus, 2 other species of large lungworms have been identified in cervids and distinguished from D. eckerti; namely D. cervi (Pyziel et al., Reference Pyziel, Laskowski, Demiaszkiewicz and Höglund2017) and D. skrjabini n. sp. (the species described in this study). The genetic variability of red deer-derived Dictyocaulus spp. has been revealed in previous studies, suggesting that several distinct species may be concealed within D. eckerti (Carreno et al., Reference Carreno, Diez-Baños, Hidalgo-Argüello and Nadler2009; Pyziel et al., Reference Pyziel, Laskowski and Höglund2015; Ács et al., Reference Ács, Hayward and Sugár2016; Cafiso et al., Reference Cafiso, Castelli, Tedesco, Poglayen, Buccheri Pederzoli, Roretto, Orusa, Corlatti, Bazzocchi and Luzzago2023). In most cases, their nucleotide sequences were homologous to D. cervi, suggesting that the species might have a wide distribution in red deer populations in Spain, Sweden, Poland, Hungary and Italy (Carreno et al., Reference Carreno, Diez-Baños, Hidalgo-Argüello and Nadler2009; Pyziel et al., Reference Pyziel, Laskowski and Höglund2015; Ács et al., Reference Ács, Hayward and Sugár2016; Cafiso et al., Reference Cafiso, Castelli, Tedesco, Poglayen, Buccheri Pederzoli, Roretto, Orusa, Corlatti, Bazzocchi and Luzzago2023). Furthermore, D. cervi has also been detected in moose in Poland (Filip-Hutsch et al., Reference Filip-Hutsch, Demiaszkiewicz, Chęcińska, Hutsch, Czopowicz and Pyziel2020), as well as in rocky mountain elk in the USA (Bangoura et al., Reference Bangoura, Brinegar and Creekmore2021). In addition, D. cervi, new genotypes of red deer lungworm have recently been detected in Italy (Cafiso et al., Reference Cafiso, Castelli, Tedesco, Poglayen, Buccheri Pederzoli, Roretto, Orusa, Corlatti, Bazzocchi and Luzzago2023).
In the present study, D. cervi was more prevalent than D. skrjabini n. sp., which accounted for only 18.7% of all positive Dictyocaulus samples from Poland. Molecular detection of D. cervi was also recorded in red deer and moose in Sweden, while D. skrjabini n. sp. was confirmed in all isolates of fallow deer and from one red deer examined. Interestingly, a new genotype of a lungworm previously found by Hӧglund et al. (Reference Höglund, Morrison, Divina, Wilhelmsson and Mattson2003) in fallow deer in Sweden showed 100% SSU rDNA homology with D. skrjabini n. sp.
The analysis of the molecular data, and their phylogenetic reconstruction revealed clear distinctions between D. cervi, D. skrjabini n. sp. and D. eckerti. Dictyocaulus skrjabini n. sp. formed independent clades in both phylogenetic trees, one based on SSU rDNA and the other on mt cytB sequences. The results further highlight the high degree of conservation within the SSU rDNA and mt cytB sequences of Dictyocaulus spp. and confirm their usefulness for systematic studies within the genus. Previous studies have indicated a high level of Dictyocaulus spp. genetic diversity within the genus at the ITS2 rDNA, and cox1, cox3 and nad5 of the mt DNA (Hӧglund et al., Reference Höglund, Morrison, Mattsson and Engström2006; Pyziel et al., Reference Pyziel, Laskowski, Demiaszkiewicz and Höglund2017; Pyziel et al., Reference Pyziel, Dolka, Werszko, Laskowski, Steiner-Bogdaszewska, Wiśniewski, Demiaszkiewicz and Anusz2018a; Pyziel et al., Reference Pyziel, Laskowski, Dolka, Kołodziej-Sobocińska, Nowakowska, Klich, Bielecki, Żygowska, Moazzami, Anusz and Höglund2020). In addition, the cox3 nucleotide sequences were found not to be suitable for lungworm species identification, as these sequences were identical for D. viviparus and D. capreolus: 2 different worm species (Pyziel et al., Reference Pyziel, Dolka, Werszko, Laskowski, Steiner-Bogdaszewska, Wiśniewski, Demiaszkiewicz and Anusz2018a). According to Blouin (Reference Blouin1998), different species of closely related nematodes can show 10–20% variation in mt gene sequences, as indicated for D. skrjabini n. sp. and D. cervi, and for D. skrjabini n. sp. and D. eckerti in the present study.
Additionally, morphological differences between D. skrjabini n. sp. and D. cervi included statistically significant discrepancies in various features.
Thus, a large lungworm found in red deer and fallow deer demonstrated clear molecular and morphological differences from previous specimens, suggesting the presence of a new species: D. skrjabini n. sp. The geographical distribution of the species requires further investigation. The exact localization of the new species in the lung is also of interest, both to better understand the drivers of speciation and to explain the pathogenicity of the parasite.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S003118202300080X.
Data availability statement
The data that support the findings of this study are available from the corresponding author (AMP).
Acknowledgements
The authors would like to express their gratitude to mgr. Edward Lowczowski for English correction of the text.
Authors’ contributions
AMP conceived and designed the study, conducted morphological and molecular investigation, wrote the article. ZL conducted phylogenetic reconstruction. DK performed statistical analysis. AD and KA supervised the study. SK, DM and JK collected the respiratory tracts of hunted red deer. JN studied the worms by scanning electron microscopy. JH supervised the study and edited the article.
Financial support
The presented data were generated during a scientific stay at the SLU supported by the Warsaw University of Life Sciences (WULS-SGGW) within the framework of the SGGW Own Scholarship Found (Własny Fundusz Stypendialny Szkoły Głównej Gospodarstwa Wiejskiego w Warszawie), decision nr. BWM – 315/2018.
Competing interest
None.
Ethical standards
The samples were taken exclusively from animals legally hunted during the 2017/2018 and 2018/2019 hunting seasons according to Polish hunting law (Act of the Polish Parliament dated 13 October 1995, Official Journal 1995, 147, item 713, the Hunting Law).