Article contents
Contrasting temperature responses in seasonal timing of cercariae shedding by Rhipidocotyle trematodes
Published online by Cambridge University Press: 16 May 2022
Abstract
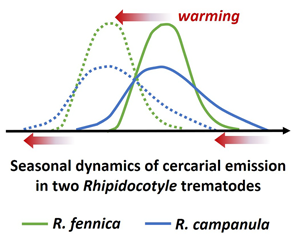
Global warming is likely to lengthen the seasonal duration of larval release by parasites. We exposed freshwater mussel hosts, Anodonta anatina, from 2 high-latitude populations to high, intermediate and low temperatures throughout the annual cercarial shedding period of the sympatric trematodes Rhipidocotyle fennica and R. campanula, sharing the same transmission pathway. At the individual host level, under warmer conditions, the timing of the cercarial release in both parasite species shifted towards seasonally earlier period while its duration did not change. At the host population level, evidence for the lengthening of larvae shedding period with warming was found for R. fennica. R. campanula started the cercarial release seasonally clearly earlier, and at a lower temperature, than R. fennica. Furthermore, the proportion of mussels shedding cercariae increased, while day-degrees required to start the cercariae shedding decreased in high-temperature treatment in R. fennica. In R. campanula these effects were not found, suggesting that warming can benefit more R. fennica. These results do not completely support the view that climate warming would invariably increase the seasonal duration of larval shedding by parasites, but emphasizes species-specific differences in temperature-dependence and in seasonality of cercarial release.
- Type
- Research Article
- Information
- Copyright
- Copyright © The Author(s), 2022. Published by Cambridge University Press
References
- 4
- Cited by



