Published online by Cambridge University Press: 30 June 2022
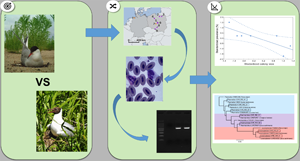
Haemoparasites represent a diverse group of vector-borne parasites that infect a wide range of vertebrate hosts. In birds, haemoparasite infection rates may be associated with various ecological and life history traits, including habitat choice, colony size and migration distance. Here, we molecularly assessed the prevalence of 3 main haemoparasite genera (Plasmodium, Haemoproteus and Leucocytozoon) in 2 bird species with different habitat preferences and migratory behaviour: black-headed gulls (Chroicocephalus ridibundus) and common terns (Sterna hirundo). We found that gulls showed a much higher prevalence and diversity of Plasmodium or Haemoproteus (ca. 60% of individuals infected) than terns (zero prevalence). The prevalence of Leucocytozoon was low in both species (<3%). The differences in haemoparasite prevalences may be primarily driven by varying vector encounter rate resulting from different habitat preferences, as black-headed gulls mainly use vector-rich vegetated freshwater habitats, whereas common terns often use vector-poor coastal and brackish habitats. Since common terns migrate further than black-headed gulls, our results did not provide support for an association between haemoparasite prevalence and migratory distance. In gulls, we found a negative association between colony size and infection rates, suggestive of an ideal despotic distribution, and phylogenetic analyses of detected haemoparasite lineages provided evidence for higher host specificity in Haemoproteus than Plasmodium. Our results suggest that the preference for coastal areas and less vegetated habitats in terns may reduce haemoparasite infection rates compared to other larids, regardless of their migratory distance, emphasizing the role of ecological niches in parasite exposure.