Introduction
Tritrichomonas foetus is a recognized reproductive parasite of cattle, causing bovine trichomoniasis, a disease classed as one of the 117 worldwide notifiable diseases by the World Organisation of Animal Health (OIE, 2018). The parasite, T. foetus, has been also isolated from the gastrointestinal track of pigs with no documented effect on the host (Šlapeta et al., Reference Šlapeta, Müller, Stack, Walker, Lew-Tabor, Tachezy and Frey2012; Mueller et al., Reference Mueller, Morin-Adeline, Gilchrist, Brown and Šlapeta2015). More recently, T. foetus ‘feline genotype’ has recognized as the cause of large bowel diarrhoea in cats (Šlapeta et al., Reference Šlapeta, Craig, McDonell and Emery2010; Gookin et al., Reference Gookin, Hanrahan and Levy2017). Cattle infection with T. foetus can cause losses of up to 70% of total farm income through a combination of clinical signs including abortion, increased calving intervals and infertility, and the requirement to prematurely cull infected bulls (Collantes-Fernandez et al., Reference Collantes-Fernandez, Mendoza-Ibarra, Pedraza-Diaz, Rojo-Montejo, Navarro-Lozano, Sanchez-Sanchez, Ruiz-Santa-Quiteria, Ortega-Mora and Osoro2014). The use of artificial insemination (AI) in intensively managed herds has reduced parasite prevalence (Bernasconi et al., Reference Bernasconi, Bodmer, Doherr, Janett, Thomann, Spycher, Iten, Hentrich, Gottstein, Müller and Frey2014). AI, however, is not practical in remote areas and hence natural mating is the mainstay of mating procedures (McCool et al., Reference McCool, Townsend, Wolfe, Simpson, Olm, Jayawardhana and Carney1988; Bernasconi et al., Reference Bernasconi, Bodmer, Doherr, Janett, Thomann, Spycher, Iten, Hentrich, Gottstein, Müller and Frey2014; Eccles, Reference Eccles2016).
Primary diagnosis of T. foetus comprises culturing followed by microscopy and quantitative polymerase chain reaction (qPCR) analysis of preputial samples (OIE Terrestrial Manual, 2012). Culturing amplifies the low number of organisms received from the preputial sample, increasing the sensitivity of microscopy and qPCR diagnosis (Mukhufhi et al., Reference Mukhufhi, Irons, Michel and Peta2003). The commercial InPouch™ TF kit has been developed in the USA, allowing for the successful transport of T. foetus cultures over long distances (Biomed Diagnostics, n.d.). This kit is unavailable in Australia and hence there is a reliance upon laboratory prepared media such as modified Diamond's medium (MDM). MDM is considered to have limited viability, with new media required after a period of 10–14 days (Levine, Reference Levine1973; Jensen, Reference Jensen1983). Furthermore, MDM requires accurate cold-chain storage at 4 °C before being warmed to 37 °C immediately prior to inoculation (Lun et al., Reference Lun, Parker and Gajadhar2000). This limits its application in testing of herds in remote areas, such as those in Northern Australia, indicating a clear requirement to increase the stability of the media used to support T. foetus monitoring and diagnosis.
This study aims to compare lyophilized MDM with standard liquid MDM to determine the impacts on the growth of T. foetus. The developed lyophilized MDM was exposed to temperature conditions reflecting those experienced in the remote areas including extended periods of heat and sunlight.
Materials and methods
Media preparation and culture maintenance
MDM (ATCC medium 719) was prepared weekly for the liquid media, or as required for lyophilization. MDM consisted of 2% BBL™ trypticase peptone (BD211921, Becton Dickinson; Australia), 1% BBL™ yeast extract (Becton Dickinson; BD211929), 0.5% Difco™ maltose (BD216830, Becton Dickinson, Australia), 0.1% L-cysteine HCl (BIOCB0132, Astral Scientific, Australia), 0.02% l-ascorbic acid (A-0278, Sigma, Australia), 0.08% di-potassium hydrogen orthophosphate (2221, Univar, Australia) and 0.08% potassium di-hydrogen orthophosphate (391, Univar, Australia). The pH was adjusted to 7.2 using 1 m NaOH and MDM then autoclaved at 121 °C for 15 min. Throughout this study, the original ATCC medium 719 preparation was modified with the addition of an antibiotic–antimycotic at 1× concentration (10 000 units/mL of penicillin, 10 000 µg/mL of streptomycin and 25 µg/mL of Gibco Amphotericin B; supplied at 100× concentration; cat. #15240062; ThermoFisher Scientific, Australia) and 5% inactivated fetal bovine serum (SFBS-F-HI, Bovogen Biologicals, Australia).
MDM was prepared in batches of 100 mL and stored at 4 °C for no more than 7 days. MDM was pre-warmed to 37 °C before inoculation with T. foetus Sydney-G10/1 (G10) that had been recovered after storage with dimethyl sulphoxide at −80 °C (Šlapeta et al., Reference Šlapeta, Craig, McDonell and Emery2010). Cultures were passaged to MDM every 2–3 days to maintain a stock culture at log growth phase at 37 °C. Throughout the study, concentrations of T. foetus were evaluated using a Neubauer counting chamber (SVZ2NIOU, ProSciTech, Australia) for a period of 5 days, using iodine diluted into a 1:1 ratio with sterile deionized water to immobilize the parasites and observed under a microscope, cells counted in five cells (corner, middle) of the large central square (20× objective, Olympus BX41, Olympus Australia).
Lyophilization of MDM
An Alpha 2-4 LSCplus freeze dryer (Martin Christ Gefriertrocknungsanlagen, GmbH, Germany) was used throughout the study for media lyophilization. Liquid media (2 mL, MDM) was dispensed into vacuum-safe vials (223738, Duran Wheaton Kimble; Edwards Group, Australia) with 2-leg grey butyl stoppers (224100-193-ED, Edwards Group, Australia) half inserted and then frozen at −80 °C, prior to lyophilization. The freeze dryer was operated manually with an initial warm-up phase, after which the frozen vials of MDM were inserted and the main dry performed over two and a half days. The machine shelf temperature was initiated at −40 °C and pressure of 0.128 mbar. The shelf temperature was raised by 10 °C every 4 h. When the shelf temperature reached 20 °C the final dry began with the pressure set to 0.005 mbar. Vials of lyophilized MDM were then stoppered under vacuum before being removed from the machine and crimped with aluminium caps (224193-01, Duran Wheaton Kimble, Edwards Group, Australia). These were stored at 24 °C (room temperature) prior to inoculation, at which time they were reconstituted with 2 mL of sterile deionized water and warmed to 37 °C.
Experiment 1 – lyophilized reconstituted vs liquid MDM viability for T. foetus
Lyophilized MDM and liquid MDM were stored under standard laboratory conditions (24 and 4 °C, respectively) prior to inoculation. The media were used immediately (0-day storage) or stored for 7, 14, 28 and 42 days.
Lyophilized MDM was reconstituted with 2 mL sterile deionized water and liquid MDM was dispensed into 2 mL aliquots and pre-warmed to 37 °C. Each medium, in triplicate, was inoculated with 104 T. foetus log-phase trophozoites and incubated at 37 °C within 1 min of inoculation. Concentrations of T. foetus were evaluated daily for 5 days post inoculation.
Experiment 2 – stability of lyophilized MDM after storage under adverse conditions
Lyophilized MDM were stored for 1 or 7 days under different combinations of conditions. Storage conditions were either 4, 10, 24, 37 or 58 °C with no light exposure (kept in a dark incubator), or 10 and 24 °C with light exposure 12:12 h light–dark cycle (100–200 µmol photons m−2 s−1).
On day 1 and day 7, lyophilized MDM was reconstituted with 2 mL sterile deionized water and pre-warmed to 37 °C. Each medium, in triplicate, was inoculated with 104 T. foetus log-phase trophozoites and incubated at 37 °C within 1 min of inoculation. Concentrations of T. foetus were evaluated daily for 5 days post inoculation.
Experiment 3 – effect of pre-inoculation temperature on T. foetus growth in reconstituted lyophilized MDM
Vials of lyophilized MDM stored (50 days) in triplicate were reconstituted with 2 mL of sterile deionized water and stored for 2 h at 4, 24 and 37 °C prior to inoculation with T. foetus. Each medium, in triplicate, was inoculated with 104 T. foetus log-phase trophozoites and incubated at 37 °C within 1 min of inoculation. Concentrations of T. foetus were evaluated daily for 5 days post inoculation.
Statistical analysis
Data were recorded, log 10 transformed then analysed and visualized using GraphPad Prism version 7 (GraphPad Software, USA). Significance was set at P < 0.05 and calculated using a Wilcoxon nonparametric test, Kruskal–Wallis nonparametric test and one-way analysis of variance (ANOVA).
Results
We tested (Experiment 1) if storage time (0–42 days) of the media (lyophilized MDM at 24 °C; liquid MDM at 4 °C) had an effect on its ability to sustain T. foetus culture (Fig. 1). For each experiment, concentrations of T. foetus were evaluated using a Neubauer counting chamber over a period of 5 days. On day 5, there was no significant difference between all treatments (Kruskal–Wallis test, P > 0.05). Individually, T. foetus yield between reconstituted lyophilized MDM and liquid MDM for day 0, 7, 14, 28 and 42 was not significant (Wilcoxon test, P > 0.05). On day 14, one triplicate of the reconstituted lyophilized MDM was a clear outlier (Fig. 1).
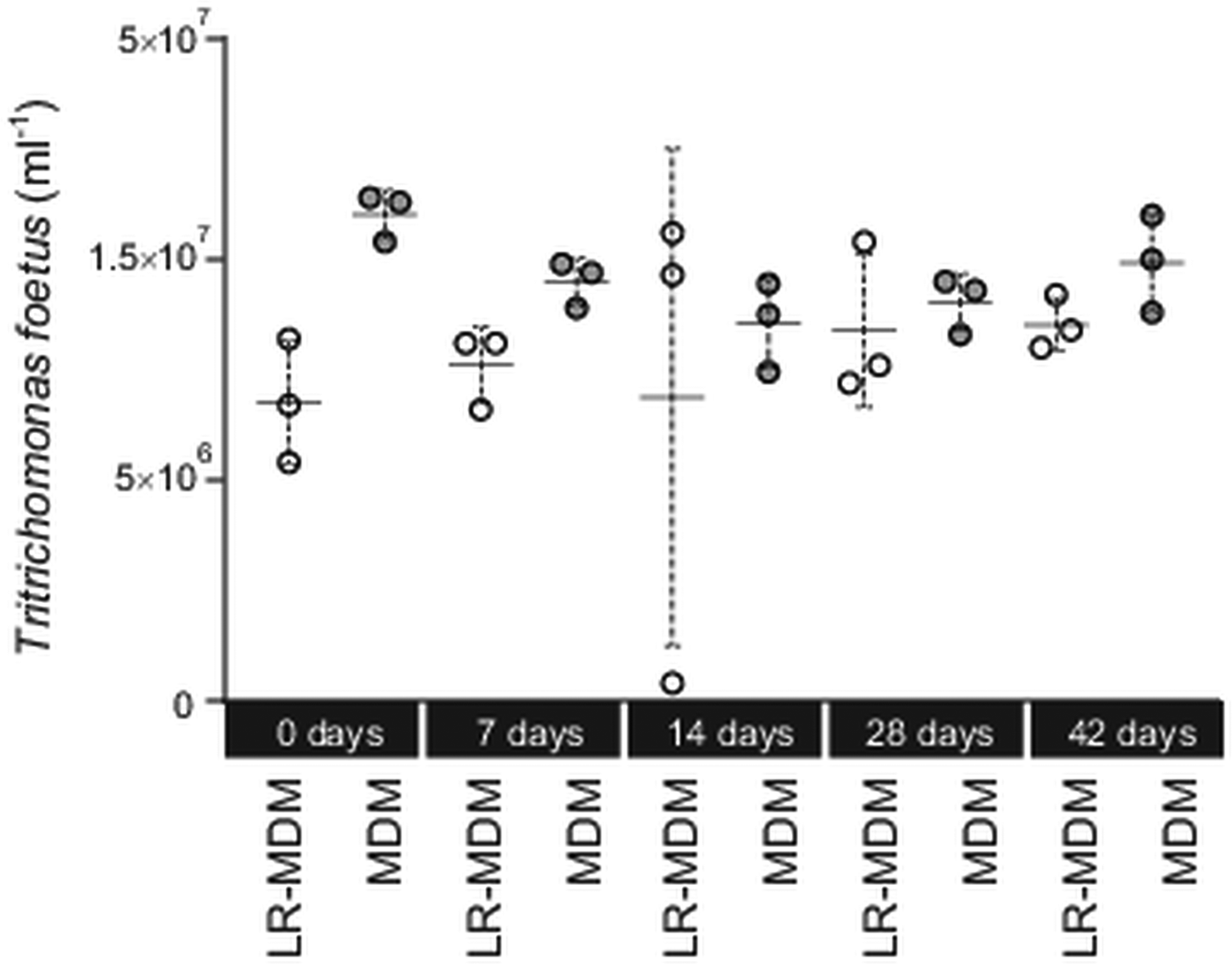
Fig. 1. Tritrichomonas foetus culture in lyophilized reconstituted MDM and liquid MDM. Prior to inoculation with T. foetus the media were stored for up to 42 days. Yield of T. foetus was recorded 5 days post inoculation for both lyophilized reconstituted MDM (LR-MDM) and liquid MDM (MDM). The scatter graph represents the triplicates inoculated for each storage time, and each form of media, with the standard deviation and mean represented for each group.
The lyophilized MDM (Experiment 2) was stored under several combinations of conditions including varying temperatures (4, 10, 24, 37 and 58 °C) and light/dark exposure for 1 and 7 days (Table 1). There was a significant difference between T. foetus yield when all conditions were compared simultaneously (Kruskal–Wallis test, P = 0.02). Excluding samples stored at 37 and 58 °C for 7 days, storage for 1 or 7 days resulted in no significant difference in T. foetus yield under other conditions (Kruskal–Wallis test, P > 0.05). Decreased yield was observed for media stored at 37 and 58 °C in the dark for 7 days compared with storage at these temperatures for 1 day (Table 1). Discoloration of the lyophilized MDM was observed in the vials stored at 37 and 58 °C for 7 days, with the lyophilized MDM stored in 58 °C partially resistant to reconstitution.
Table 1. Effect of storage condition of lyophilized MDM on Tritrichomonas foetus culture
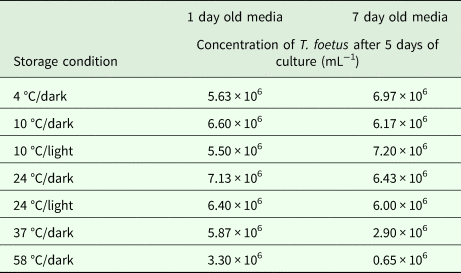
Each concentration is an average of three replicates.
Experiment 3 demonstrated that pre-inoculation temperatures of 4, 24 and 37 °C of reconstituted lyophilized MDM had no significant effect on the yield of T. foetus (log transformed, one-way ANOVA, P > 0.05). Data for this experiment are not shown.
Discussion
This study trialled the use of lyophilization to address the limitations of liquid MDM to allow for transport and T. foetus diagnosis in extensively managed cattle herds. The approach addressed the limiting stability and cold chain requirements of MDM, which reduce its ability to support the culture and diagnosis of T. foetus. The study has demonstrated that reconstituted lyophilized MDM is equally comparable with liquid MDM and is able to retain viability at room temperature for 6 weeks (42 days, Experiments 1 and 2) and under conditions reflecting those in remote areas.
The use of MDM for culture of T. foetus was implemented in the late 1950s (Levine, Reference Levine1973). Early methods supplement the media with sheep or horse serum and state the medium should not be utilized past 10–14 days of refrigeration due to loss of viability (Levine, Reference Levine1973; Jensen, Reference Jensen1983). Our study demonstrates the capacity of MDM supplemented with 5% FBS, which was consistently able to support T. foetus culture up to 42 days when stored at 4 °C. FBS is considered as superior to other forms of serum (Marco-Jimenez et al., Reference Marco-Jimenez, Garzon, Penaranda, Perez, Viudes-de-Castro, Vicente, Jover and Asturiano2006; Nims and Harbell, Reference Nims and Harbell2017). The use of FBS in cell culture lines decreases the doubling time of cells and significantly increases the number of possible passages when compared to horse serum (Nims and Harbell, Reference Nims and Harbell2017). The addition of 5% FBS to liquid MDM is beneficial and enables storage of media for up to 6 weeks for T. foetus culture. Fetal calf serum is also recommended for T. foetus transport (Buller and Corney, Reference Buller and Corney2013).
Lyophilization is utilized for its ability to reduce the required maintenance and storage conditions of many substances including vaccines, biological materials and food (Tang and Pikal, Reference Tang and Pikal2004; Bjelošević et al., Reference Bjelošević, Selja, Trstenjak, Logar, Brus and Grabnar2018). The process has been demonstrated in parasitology, whereby amoebae have been cultured and then lyophilized for storage, reducing laborious requirements associated with maintenance of the liquid culture (Balczun and Scheid, Reference Balczun and Scheid2018). For stability during storage there is a requirement to know the conditions conducive to long-term stable preservation whilst retaining the ability to reconstitute viable cells or material (Holzer et al., Reference Holzer, Vogel, Mantele, Schwartz, Haase and Langer2009; Balczun and Scheid, Reference Balczun and Scheid2018). In this study, the developed lyophilized MDM was tested under pre-inoculation storage conditions outside the scope of liquid MDM, primarily focussing on storage at temperatures reflecting those in remote tropical areas. Our results demonstrate that lyophilized MDM is capable of retaining viability in temperatures ranging from 4 to 24 °C. This result is consistent with what is expected for all lyophilized products, whereby storage in ambient temperatures results in the least changes to the molecular structure of the lyophilized product (Holzer et al., Reference Holzer, Vogel, Mantele, Schwartz, Haase and Langer2009). Storage at high temperatures includes exposure to high levels of humidity and hence moisture, resulting in a loss of stability (Duralliu et al., Reference Duralliu, Matejtschuk and Williams2018).
The labour and financial costs associated with mustering, holding and sampling of large, extensive herds is the biggest limitation for the diagnosis of T. foetus in Australia (McCool et al., Reference McCool, Townsend, Wolfe, Simpson, Olm, Jayawardhana and Carney1988). Whilst the lyophilization process is costly, it is a viable alternative to InPouch™ TF (Biomed Diagnostics, n.d.) and presents a form of medium that can be maintained on-site with instructions as to how media should be reconstituted, and samples retrieved, packaged and sent. Preputial samples are often collected using a Tricamper™, which may be rinsed into culture medium (Buller and Corney, Reference Buller and Corney2013). Variability of rinsing procedures has been a factor influencing sensitivity and specificity of T. foetus testing (Cobo et al., Reference Cobo, Favetto, Lane, Friend, VanHooser, Mitchell and BonDurant2007). Preparation of lyophilized media in vials allowing the full immersion of a Tricamper™ tip would potentially decrease the variability. Improvements in T. foetus sampling techniques associated with the lyophilized MDM would allow for a wide-scale survey more representative of the 12.7 million head of cattle residing in northern Australia (National Farmer's Federation, 2017). The stability of lyophilized MDM during storage and transport will allow incorporation of T. foetus testing into yearly husbandry procedures, with collections performed during routine annual animal mustering. To assist with accurate reconstitution of the lyophilized MDM, a kit would be provided that would include a 2 mL syringe for each vial, ensuring volume and hence medium concentration is consistent. The availability of media for T. foetus without the need of a continuous cold chain provides the basis for improvement of extensive industry diagnosis of bovine trichomoniasis.
Author ORCIDs
Michael William Reynolds, 0000-0002-6592-7232; Nichola Eliza Davies Calvani, 0000-0002-8787-6755; Jan Šlapeta, 0000-0003-1459-9117
Acknowledgements
We would like to acknowledge Katrina Gilchrist (Sydney School of Veterinary Science) for her assistance with laboratory work.
Financial support
The work was in part supported by the McGarvie Smith Institute Honours Scholarship (GR) and Faculty of Science Honours Fund (GR).
Conflict of interest
The authors have no conflicts of interest to declare.
Ethical standards
Not applicable.





