Published online by Cambridge University Press: 04 March 2022
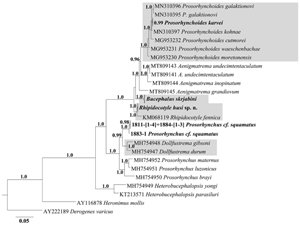
Morphological data and the first molecular data are provided for four species of the trematode family Bucephalidae Poche, 1907 from marine and freshwater teleost fish species of East Asia. A new species, Rhipidocotyle husi n. sp., was isolated from Huso dauricus from the Amur River, Russia. Adult worms of this species were distinguished from their congeners Rhipidocotyle illense and Rhipidocotyle kovalai by morphological analysis. Three other known species were identified: Bucephalus skrjabini and Prosorhynchus cf. squamatus were detected in Siniperca chuatsi from the Amur River and in Myoxocephalus spp. from the Okhotsk Sea, Russia, respectively, while Prosorynchoides karvei was extracted from Strongylura strongylura from Halong Bay, Vietnam. The 28S ribosomal DNA (rDNA)-based phylogenetic analysis showed that the new species formed a shared polytomy clade with Rhipidocotyle fennica. Phylogenetic analysis of all available molecular data showed that four genera, namely Rhipidocotyle, Bucephalus, Prosorynchoides and Prosorhynchus, are para- or polyphyletic. Molecular-based phylogenetic analysis of morphologically validated bucephalid species indicated that three genera – Rhipidocotyle, Bucephalus and Prosorynchoides – were monophyletic. The genus Prosorhynchus maintained paraphyly, and P. cf. squamatus was more closely related to Dollfustrema spp. than to other Prosorhynchus spp. These findings do not exclude the possibility that representatives of Dollfustrema and P. cf. squamatus belong to the same genus.