Article contents
Quantitative monitoring of experimental and human leishmaniasis employing amastigote-specific genes
Published online by Cambridge University Press: 10 May 2022
Abstract
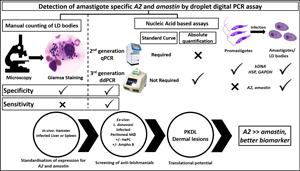
The gold standard for diagnosis of leishmaniasis is the microscopic detection of amastigotes/Leishman Donovan (LD) bodies, but its moderate sensitivity necessitates the development of molecular approaches. This study aimed to quantify in experimental animal models and human leishmaniasis the expression of amastigote-specific virulence genes, A2 and amastin by droplet digital polymerase chain reaction (ddPCR). Total RNA was isolated from L. donovani-infected hamsters or murine peritoneal macrophages and lesional biopsies from patients with post kala-azar dermal leishmaniasis (PKDL). Following cDNA conversion, EvaGreen-based ddPCR was performed using specific primers for A2 or amastin and parasite load expressed in copies per μL. Assay was optimized and the specificity of amastigote-specific A2 and amastin was confirmed. In hepatic and splenic tissues of L. donovani-infected hamsters and peritoneal macrophages, ddPCR demonstrated a greater abundance of A2 than amastin. Treatment of L. donovani-infected peritoneal macrophages with conventional anti-leishmanials, miltefosine and amphotericin B translated into a dose-dependent reduction in copies per μL of A2 and amastin, and the extrapolated IC50 was comparable with results obtained by counting LD bodies in Giemsa-stained macrophages. Similarly, in dermal biopsies of patients with PKDL, A2 and amastin were detected. Overall, monitoring of A2 by ddPCR can be an objective measure of parasite burden and potentially adaptable into a high throughput approach necessary for drug development and monitoring disease progression when the causative species is L. donovani.
Keywords
- Type
- Research Article
- Information
- Copyright
- Copyright © The Author(s), 2022. Published by Cambridge University Press
References
- 4
- Cited by



