Article contents
Helminth parasites are associated with reduced survival probability in young red deer
Published online by Cambridge University Press: 02 September 2022
Abstract
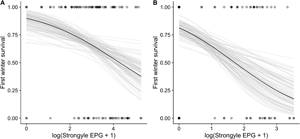
Helminths are common parasites of wild ungulates that can have substantial costs for growth, mortality and reproduction. Whilst these costs are relatively well documented for mature animals, knowledge of helminths' impacts on juveniles is more limited. Identifying these effects is important because young individuals are often heavily infected, and juvenile mortality is a key process regulating wild populations. Here, we investigated associations between helminth infection and overwinter survival in juvenile wild red deer (Cervus elaphus) on the Isle of Rum, Scotland. We collected fecal samples non-invasively from known individuals and used them to count propagules of 3 helminth taxa (strongyle nematodes, Fasciola hepatica and Elaphostrongylus cervi). Using generalized linear models, we investigated associations between parasite counts and overwinter survival for calves and yearlings. Strongyles were associated with reduced survival in both age classes, and F. hepatica was associated with reduced survival in yearlings, whilst E. cervi infection showed no association with survival in either age class. This study provides observational evidence for fitness costs of helminth infection in juveniles of a wild mammal, and suggests that these parasites could play a role in regulating population dynamics.
- Type
- Research Article
- Information
- Copyright
- Copyright © The Author(s), 2022. Published by Cambridge University Press
References
- 5
- Cited by



