No CrossRef data available.
Published online by Cambridge University Press: 15 June 2022
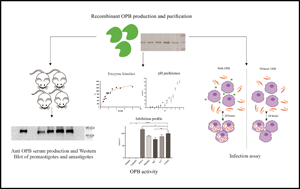
Leishmania spp. are parasitic protozoa that cause leishmaniasis, a disease endemic in 98 countries. Leishmania promastigotes are transmitted by the vector and differentiate into amastigotes within phagocytic cells of the vertebrate host. To survive in multiple and hostile environments, the parasite has several virulence factors. Oligopeptidase B (OPB) is a serine peptidase present in prokaryotes, some eukaryotes and some higher plants. It has been considered a virulence factor in trypanosomatids, but only a few studies, performed with Old World species, analysed its role in Leishmania virulence or infectivity.
L. (L.) amazonensis is an important agent of cutaneous leishmaniasis in Brazil. The L. (L.) amazonensis OPB encoding gene has been sequenced and analysed in silico but has never been expressed. In this work, we produced recombinant L. (L.) amazonensis OPB and showed that its pH preferences, Km and inhibition patterns are similar to those reported for L. (L.) major and L. (L.) donovani OPBs. Since Leishmania is known to secrete OPB, we performed in vitro infection assays using the recombinant enzyme. Our results showed that active OPB increased in vitro infection by L. (L.) amazonensis when present before and throughout infection. Our findings suggest that OPB is relevant to L. (L.) amazonensis infection, and that potential drugs acting through OPB will probably be effective for Old and New World Leishmania species. OPB inhibitors may eventually be explored for leishmaniasis chemotherapy.