Introduction
Members of the genus Trypanosoma belong to the family Trypanosomatidae and present a complex taxonomic classification due to their wide morphological, biological and molecular variation. Haematophagous arthropods act as biological or mechanical vectors for different species of this family, infecting a wide range of vertebrate hosts. Several species of the genus Trypanosoma are aetiological agents of diseases transmitted to humans and animals, stimulating interest in these protozoans (Hoare, Reference Hoare1972; Haag et al. Reference Haag, O'HUigin and Overath1998).
While most Trypanosoma species are transmitted by blood-sucking insects, ticks are also likely to be vectors of some members of this genus (Morzaria et al. Reference Morzaria, Latif, Jongejan and Walker1986; Thekisoe et al. Reference Thekisoe, Honda, Fujita, Battsetseg, Hatta, Fujisaki, Sugimoto and Inoue2007). We recently reported isolation of a novel trypanosome, Trypanosoma rhipicephalis sp. nov, from Brazilian Rhipicephalus microplus ticks removed from cattle (Marotta et al. Reference Marotta, Santos, Cordeiro, Matos, Barros, Madeira, Bell-Sakyi and Fonseca2018).
This study describes an isolate of the genus Trypanosoma naturally infecting Amblyomma brasiliense ticks parasitizing the white-lipped peccary Tayassu pecari, characterized through molecular, morphological and biological analyses. Although A. brasiliense are aggressive towards humans, their vector capacity for aetiologic agents of diseases transmitted to humans or animals is unknown (Aragao, Reference Aragao1936; Sanches et al. Reference Sanches, Bechara, Garcia, Labruna and Szabo2008).
Materials and methods
Origin of A. brasiliense ticks
A specimen of adult T. pecari was found dead in the Itaporani Waterfall, Itatiaia National Park, Itatiaia, Rio de Janeiro, Brazil (located between the coordinates 22°19′ and 22°45′S, and 44°15′ and 44°50′W). The animal was kept refrigerated (2–8 °C) for approximately 24 h and was sent to the Federal Rural University of Rio de Janeiro (UFRRJ) municipality of Seropedica, state of Rio de Janeiro for post-mortem investigation. The necropsy report confirmed that the macroscopic findings were consistent with vertebral fractures and cavitary haemorrhages, consistent with trauma, probably due to a fight between animals and fall into a waterfall.
Eight live ticks were collected from the peccary and identified according to Barros-Battesti et al. (Reference Barros-Battesti, Arzua and Bechara2006) for adults and Martins et al. (Reference Martins, Onofrio, Barros-Battesti and Labruna2010) for nymphs. Nymphs and adults were identified as belonging to the species A. brasiliense. It was not possible to identify the larvae to the species level.
Isolation of trypanosomes
In a laminar flow cabinet, live ticks were surface-sterilized by immersion in 70% ethanol for 1 min, 0.05% sodium hypochlorite solution for 30 s, 70% ethanol again for 1 min, detergent based on 2% chlorhexidine (Riohex® Rioquimica, Brazil) for 30 s, a third wash in 70% ethanol for 1 min and finally sterile ultrapure water with penicillin (100 IU mL−1), streptomycin (100 µg mL−1) and amphotericin B (250 µg mL−1) for 1 min. The ticks were then dried on sterile gauze.
After surface sterilization, the ticks were separated into two pools, consisting of one pool of four Amblyomma sp. larvae and one pool of two nymphal and two adult A. brasiliense. The pooled ticks were crushed in a beaker with the aid of a glass syringe plunger (piston). The crushed ticks were resuspended in 5 mL of L15B medium (Munderloh and Kurtti, Reference Munderloh and Kurtti1989) supplemented with 10% heat-inactivated fetal calf serum (FCS), 10% tryptose phosphate broth, 0.1% bovine lipoprotein concentrate (MP Biomedicals, United Kingdom), 2 mm L-glutamine, 100 IU mL−1 penicillin and 100 µg mL−1 streptomycin, pH adjusted to 6.6–6.8 with 1N NaOH.
Each tick suspension, containing all parts of the ticks without prior centrifugation, was transferred to a 25 cm2 flask containing a monolayer of cells of the Ixodes scapularis embryo-derived cell line IDE8 (Munderloh et al. Reference Munderloh, Liu, Wang, Chen and Kurtti1994) 4 days after seeding at passage 112, grown in complete L15B medium as described above, and incubated at 32 °C.
Maintenance and monitoring of trypanosome cultures
The inoculated cultures were monitored by examination of Giemsa-stained smears, prepared by spreading a drop of culture supernatant on a slide and air drying, every 3 days post-infection (DPI). Following initial isolation in IDE8 cells, trypanosome cultures were maintained in two ways: co-cultivation with IDE8 cells and axenic culture in complete L15B medium, both incubated in sealed 25 cm2 culture flasks in a bacteriological incubator at 30 °C. Renewal of the medium from cultures with IDE8 cells was performed weekly by removal and replacement of approximately 2/3 of the medium.
To obtain a pure, tick cell-free trypanosome culture after four passages in IDE8 cultures, the isolated trypanosomes were resuspended, collected by rinsing and transferred to a sterile 15 mL tube for centrifugation at 700×g for 10 min. The supernatant was transferred to a new sterile 15 mL tube and centrifuged at 200×g for 10 min. Then the supernatant was discarded and the pellet was resuspended in 8 mL of phosphate-buffered saline and examined by inverted microscope to rule out the presence of tick cells. After further centrifugation at 200×g for 10 min, the resultant pellet was resuspended in 5 mL of complete L15B medium, transferred to a 25 cm2 culture flask and incubated at 30 °C. The culture medium was renewed weekly, with removal and replacement of approximately 2/3 of the medium after mixing of the culture. Cultures were monitored with an inverted phase contrast microscope and by examination of Giemsa-stained smears of culture supernatant prepared as above.
The isolated trypanosomes were passaged by transfer of 1 mL of culture supernatant (1 : 5 dilution) to three replicates each of both IDE8 cultures and axenic cultures in 25 cm2 flasks. Aliquots of the axenically cultured trypanosomes were cryopreserved with 10% DMSO in liquid nitrogen at −196 °C at monthly intervals following isolation. For freezing, a culture was resuspended and transferred to a 15 mL sterile centrifuge tube and centrifuged at 500×g for 5 min. Thereafter, the supernatant was removed and the pellet resuspended in 2 mL of complete L15B culture medium. An equal volume of ice-cold culture medium with 20% filtered DMSO was added dropwise and gently mixed. The cell suspension was divided between four labelled cryotubes, placed in a Nalgene™ Cryo 1 °C isopropanol freezing container and held at −80 °C for at least 90 min. Subsequently the cryotubes were transferred to a liquid nitrogen storage container (−196 °C). The cryopreserved trypanosomes were resuscitated 2 months after freezing by removal from liquid nitrogen, thawed slowly in a water bath at 32 °C, diluted in 5 mL complete L15B medium and incubated at 30 °C.
Propagation in different culture conditions
Axenic propagation of the isolated trypanosomes was tested in the following culture media: MEM and DMEM (supplemented with 2 mm L-glutamine and 10% fetal bovine serum), M199 (supplemented with 10% fetal bovine serum), BHI, BHI supplemented with blood agar, and Schneider's insect medium (supplemented with 10% fetal bovine serum). Axenic propagation was tested at the following incubation temperatures over 15 days: 26, 28, 30, 32, 34 and 37 °C. Three replicate cultures in 25 cm2 flasks were evaluated for each condition.
Growth profile and developmental forms in axenic culture
The developmental profile of the isolated trypanosomes in axenic culture was evaluated at an early passage, 4 days after subculture. Initially, the viable (highly motile) trypanosomes were counted in a Neubauer chamber to prepare the inoculum concentration of 1 × 104 parasites mL−1 and were subsequently transferred to axenic cultures in 25 cm2 culture flasks with complete L15B medium. The growth curve was performed in triplicate at 30 °C. Aliquots of 10 µL were collected at intervals of 48 h until the 30th DPI for quantification as above and morphological analysis until the 16th DPI. Developmental forms were analysed in Giemsa-stained smears by examination of 50–100 trypanosomes per sample, based on published descriptions of Barros et al. (Reference Barros, Fonseca, Macedo-Silva, Côrte-Real, Toma and Madeira2014). No medium change was performed during this 30-day period.
Morphometric analysis
Morphometry was performed on randomly selected stained trypanosomes from axenic cultures, evaluated with a light microscope (Olympus BX45®) coupled with a photo documentation system (D'Cell®software). The measurements were performed according to Hoare (Reference Hoare1972), by evaluating the total length of the parasite (from the anterior end to the posterior end), the free flagellum length, nucleus diameter, kinetoplast diameter, distance from the posterior end to the middle of the nucleus, distance from the posterior end to the middle of the kinetoplast, distance from the middle of the nucleus to the middle of the kinetoplast, distance from the middle of the nucleus to the anterior end.
DNA extraction and polymerase chain reaction
DNA extraction was performed on trypanosomes at the second passage in axenic culture, using a Qiagen® Qiamp kit according to the manufacturer's recommendations.
Nested-polymerase chain reaction (PCR) was performed for amplification of a partial region of the 18S rDNA gene specific to the family Trypanosomatidae using TRY927F (5′-GAAACAAGAAACACGGGAG-3′) and TRY927R (5′-CTACTGGGCAGCTTGGA-3′) external primers that amplify a fragment of approximately 900 bp, and SSU561F (5′-TGGGATAACAAAGGAGCA-3′) and SSU561R (5′-CTGAGACTGTAACCTCAAAGC-3′) internal primers that amplify a fragment of approximately 700 bp, according to the protocol of Smith et al. (Reference Smith, Clark, Averis, Lymbery, Wayne, Morris and Thompson2008).
To amplify the partially conserved sequence of the largest ribosomal subunit gene (24Sα rDNA) of members of the family Trypanosomatidae, PCR was performed using D75 (5′-GCAGATCTTGGTTGGCGTAG-3′) and D76 (5′-GGTTCTCTGTTGCCCCTTTT-3′) primers that amplify a fragment of approximately 270 bp, according to Souto et al. (Reference Souto, Vargas and Zingales1999).
PCR products were subjected to 2% agarose gel electrophoresis at 90 W for 30 min. The gels were stained with ethidium bromide and visualized with a UV light transilluminator.
Sequencing and phylogenetic analysis
PCR amplification products were purified using the QIAquick® PCR Purification Kit (Qiagen), according to the manufacturer's recommendations. After purification, the DNA was sequenced using a capillary-type Sanger platform in an ABI 3730 DNA Analyser (Applied Biosystems, Life Technologies®). The resultant sequences were compared with the published sequences using the NCBI Nucleotide BLAST program.
Phylogenetic trees were built from the partial sequences of the 18S rRNA gene using the Mega 6 program, the Maximum Likelihood test and the Tajima–Nei model.
Results
Trypanosome isolation and culture
The crushed tick suspension did not have any adverse effect on the IDE8 cells detectable by microscopic examination. No microorganisms were detected in the culture inoculated with material from the four larval ticks. From the pool of four A. brasiliense nymphal and adult ticks inoculated into IDE8 cell culture, typical forms of Trypanosoma were seen from the seventh DPI onwards. The isolated trypanosomes, designated strain C1RJ, grew well axenically in complete L15B medium at temperatures of 30, 32 and 34 °C but not at higher or lower temperatures. However, no parasite growth and no viable trypanosomes were seen after the seventh DPI in the other culture media tested (MEM, DMEM, M199, BHI, BHI supplemented with blood agar, Schneider's insect medium) at all temperatures examined.
The trypanosome culture remained viable over 24 passages in axenic culture in L15B medium, and was co-cultured with IDE8 cells through ten passages, both at 30 °C. Co-cultivation of the trypanosomes with IDE8 cells resulted in detachment and subsequent death of the tick cells, commencing at seven DPI. Trypanosomes cryopreserved at the third axenic passage were viable when resuscitated after 60 days of storage in liquid nitrogen. Figure 1A shows the growth curve obtained in axenic culture in L15B at 30 °C over a 30-day period. The growth curve was initiated with an inoculum (day 0) of 1 × 104 parasites mL−1 that comprised 87% typical epimastigote forms with elongated bodies, well-tapered posterior and anterior ends, 12% trypomastigotes with an average body length smaller than the epimastigote form and kinetoplast posterior to the nucleus and 1% spheromastigote forms (Fig. 1B). Epimastigote forms predominated during the first fortnight, with a fall to below 50% and a concurrent increase in spheromastigote forms seen on the 16th DPI. Peak growth was seen on the 16th DPI with 910 × 104 parasites/mL. By the 30th DPI, degenerating and dead forms predominated (data not shown).
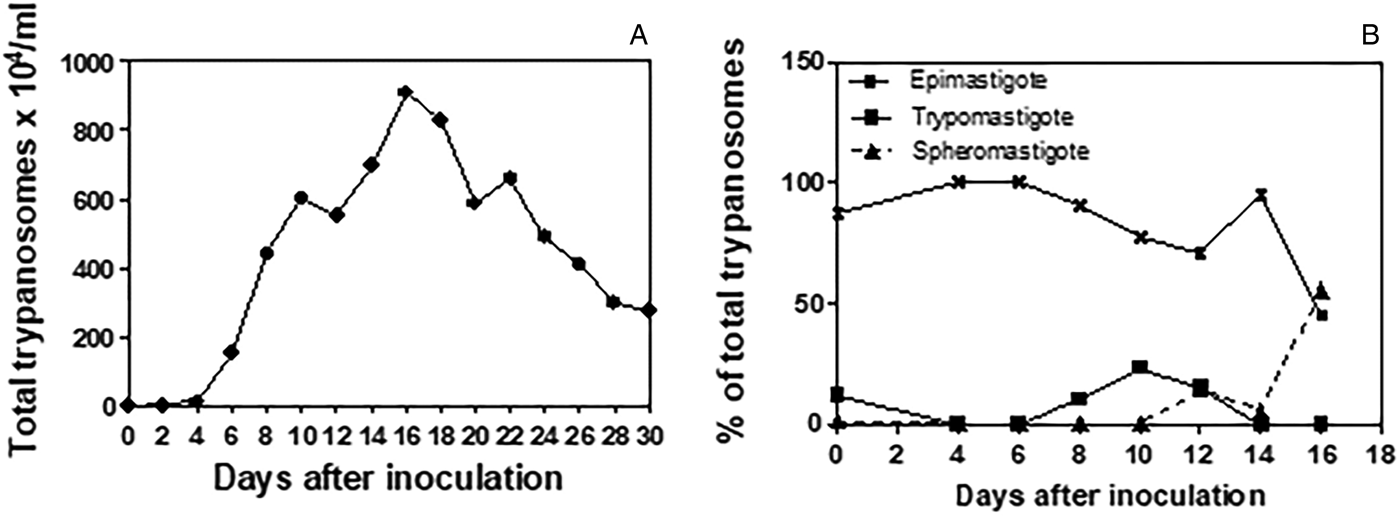
Fig. 1. Growth of Trypanosoma amblyommi sp. nov. in axenic culture in complete L15B medium. (A) Growth curve determined by counting total numbers of trypanosomes at 2-day intervals over 30 days; mean of three replicate cultures. (B) Proportions of different developmental forms determined by the examination of Giemsa-stained smears prepared at 2-day intervals over 16 days.
Morphometric analysis
Morphometric variations between different developmental forms in axenic culture were observed (Fig. 2). The morphometric measurements of trypanosome strain C1RJ trypomastigote, epimastigote and spheromastigote developmental forms are presented in Table 1.

Fig. 2. Photomicrographs showing morphological diversity of Trypanosoma amblyommi sp. nov. in axenic culture in complete L15B medium at 30 °C. (A) spheromastigote; (D, F) dividing form; (E) epimastigote; (B, G, C) forms in transition to trypomastigote Giemsa-stained smears; scale bar = 20 µ m.
Table 1. Morphometric data (μ m) obtained from trypomastigote, epimastigote and spheromastigote developmental forms of Trypanosoma amblyommi sp. nov.
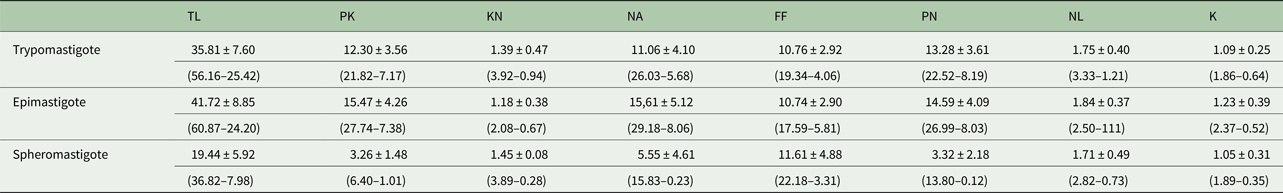
PK, posterior end to kinetoplast; KN, kinetoplast to middle of nucleus; PN, posterior end to middle of nucleus; NA, middle of nucleus to anterior end; FF, free flagellum; TL, total length; NL, nucleus diameter; K, kinetoplast diameter. Average ± standard deviation (minimum–maximum value).
The epimastigote form showed the greatest average body length (41.72 µ m) and the spheromastigote form had the lowest mean total body length (19.44 µ m). The mean length of the free flagellum was similar between the epimastigote and trypomastigote forms, with the spheromastigote form having the largest free flagellum length (11.61 µ m). The diameters of the nucleus and kinetoplast of the epimastigote, trypomastigote and spheromastigote forms were not significantly different. The mean distance between the posterior end and middle of the nucleus was similar between the epimastigote and trypomastigote forms, but much shorter in the spheromastigote form (3.32 µ m). The mean distance from the middle of the nucleus to the middle of the kinetoplast was similar in the epimastigote and spheromastigote forms. The epimastigote form presented the greatest mean values for the distance from the posterior extremity to the middle of the kinetoplast (15.47 µ m) and the distance from the anterior end to the middle of the nucleus (15.61 µ m). The diameter of the nucleus was smallest in the spheromastigote form (1.71 µ m).
Molecular analysis
In the nested-PCR carried out on axenically cultured trypanosomes, the 18S rDNA PCR fragment was approximately 900 bp in the first round of amplification. In the second round, the size of the amplified fragment was approximately 700 bp. The partial sequence of the 18S rDNA gene showed 89% similarity with T. rhipicephalis (accession number KX711901) matching 99% of the query sequence, and 88% similarity with Trypanosoma KG1 (accession number AB281091) matching 99% of the query sequence. It also presented 90% similarity (with e-value 2 e-133) and 60% alignment with Trypanosoma caninum (accession numbers JF951431, JF9075537). In the 24Sα rDNA PCR reaction using primers D75 and D76, the size of the amplified fragment was 270 bp for the isolate. For the 24Sα rDNA gene, there was 96% similarity with Trypanosoma rangeli (query coverage 63% GenBank KJ742907), Trypanosoma grosi AKHA (query coverage 65% GenBank AB175624). The phylogenetic analysis targeting the 18S rDNA gene (sequence conserved within the family Trypanosomatidae) confirmed that the trypanosome isolated from A. brasiliense belongs to the family Trypanosomatidae. The phylogenetic tree showed that the species is within the same clade as T. rhipicephalis and in a clade close to T. caninum and Trypanosoma KG1 (Fig. 3). The analysis of the 18S rDNA region confirmed the authenticity of this new species. Molecular analysis showed that our trypanosome isolate, strain C1RJ, was clearly separated from other species of the genus Trypanosoma, regardless of the molecular target used, with bootstrap values of 85 for the tree built using the target 18S rDNA sequence (Fig. 3). The nucleotide sequences described were deposited in GenBank under the access number KX711902.

Fig. 3. Phylogenetic analysis of Trypanosoma amblyommi sp. nov. and other trypanosome species. Phylogenetic tree based on 18S rDNA sequence analysis. Statistical method Maximum Likelihood – Kimura two-parameter model. Bootstrap: 1000.
Discussion
Here we describe the first isolation, molecular characterization, morphological and biological analyses of a member of the genus Trypanosoma infecting ticks of the species A. brasiliense parasitizing a specimen of T. pecari, from the municipality of Itatiaia, RJ, Brazil. The new species was named Trypanosoma amblyommi sp. nov.
The A. brasiliense tick is endemic in South America, with reports in Argentina, Paraguay and Brazil (Guglielmone et al. Reference Guglielmone, Peña, Keirans and Robbins2003; Sanches et al. Reference Sanches, Bechara, Garcia, Labruna and Szabo2008). Humans are often parasitized by this species of tick in Brazil (Aragao, Reference Aragao1936), but their vectorial capacity for bioagents infecting humans or animals is still unknown (Sanches et al. Reference Sanches, Bechara, Garcia, Labruna and Szabo2008). The trypanosome isolated in the present study was found in a pool of nymphs and adults of A. brasiliense, which are among the most aggressive ticks attacking humans in Brazil (Aragao, Reference Aragao1936).
Despite the isolation of this Trypanosoma from crushed ticks, it is not possible to determine whether the origin of the protozoan was from a vertebrate or invertebrate host.
The T. pecari specimen presented a good body score and high infestation by ticks. The autopsy report confirmed trauma with macroscopic findings consistent with vertebral fractures and internal bleeding. There was no evidence of injury caused by trypanosomatid infection. In Brazil, wild pigs were identified as important reservoirs of Trypanosoma evansi and Trypanosoma cruzi (Herrera et al. Reference Herrera, Abreu, Keuroghilian, Freitas and Jansen2008).
Some species of the genus Trypanosoma multiply intracellularly in the vertebrate host in the amastigote form, e.g. T. cruzi, in blood in the trypomastigote form, e.g. Trypanosoma brucei or in the epimastigote form, e.g. species of the subgenus Megatrypanum (Hoare, Reference Hoare1972). In the present study, in vitro replication of T. amblyommi was in the epimastigote form.
An interesting observation was the isolation using the tick cell line IDE8 and the inability of T. amblyommi to grow in conventional trypanosome culture media and at conventional incubation temperatures. Trypanosoma amblyommi was propagated in L15B medium, which is used for culturing tick cells (Munderloh and Kurtti., Reference Munderloh and Kurtti1989) and only at temperatures between 30 and 34 °C. Similar behaviour was observed for the recently described T. rhipicephalis, also isolated into IDE8 cell culture from ticks in the state of Rio de Janeiro (Marotta et al. Reference Marotta, Santos, Cordeiro, Matos, Barros, Madeira, Bell-Sakyi and Fonseca2018). In the present study, although no adverse effect of the initial crushed tick suspension on the IDE8 cells was detected, subsequent co-cultivation of T. amblyommi with tick cells resulted in detachment and death of the latter. Further studies are required to establish whether the tick cell death resulted simply from competition with the faster growing trypanosomes for nutrients in the culture medium, from release of toxic substances by the trypanosomes, or because trypanosomes were internalized by the tick cells and somehow compromised the latter's viability. This phenomenon was not reported to occur with T. rhipicephalis in IDE8 cells (Marotta et al. Reference Marotta, Santos, Cordeiro, Matos, Barros, Madeira, Bell-Sakyi and Fonseca2018).
The morphometric evaluation revealed wide morphological diversity. Trypanosoma amblyommi presented large dimensions, as seen by the total length of the trypomastigote form with a mean of 35.81 µ m, being larger than Trypanosoma vivax (Ramírez et al. Reference Ramírez, Dávila, Victório, Silva, Trajano and Jansen1997), T. cruzi (Madeira et al. Reference Madeira, Sousa, Barros, Figueiredo, Fagundes, Schubach, De Paula, Faissal, Fonseca, Thoma and Marzochi2009), T. evansi (Elshafie et al. Reference Elshafie, Sani, Hassan, Sharma, Bashir and Abubakar2013) and T. rangeli (Madeira et al. Reference Madeira, Sousa, Barros, Figueiredo, Fagundes, Schubach, De Paula, Faissal, Fonseca, Thoma and Marzochi2009), but smaller than Trypanosoma theileri (Wink, Reference Wink1979) and T. caninum (Madeira et al. Reference Madeira, Sousa, Barros, Figueiredo, Fagundes, Schubach, De Paula, Faissal, Fonseca, Thoma and Marzochi2009). Wide variation was also observed in other measurements, such as the distance between the posterior end and the kinetoplast, the distance between the nucleus and anterior end and the posterior end to the nucleus.
Sequencing analysis of the 18S rDNA and 24Sα rDNA regions confirmed the authenticity of this new species. In the phylogenetic 18S rDNA analysis, T. amblyommi appears within the same clade as T. rhipicephalis, T. caninum and Trypanosoma KG1. Trypanosoma KG1 was described after isolation from naturally infected Haemaphysalis hystricis ticks in Japan (Thekisoe et al. Reference Thekisoe, Honda, Fujita, Battsetseg, Hatta, Fujisaki, Sugimoto and Inoue2007), while T. caninum was isolated from a dog in Brazil (Madeira et al. Reference Madeira, Sousa, Barros, Figueiredo, Fagundes, Schubach, De Paula, Faissal, Fonseca, Thoma and Marzochi2009) and its vector is unknown.
Our results indicate that T. amblyommi is a new species of the genus Trypanosoma. However, aspects related to ultrastructure, pathogenicity, involvement with vertebrate hosts, epidemiology, cycle, transmission mechanisms, classification and taxonomy are still unknown. Further studies are needed to determine these aspects of the biological cycle of the newly identified T. amblyommi. Isolation in the present study of T. amblyommi and, using similar techniques, of T. rhipicephalis (Marotta et al. Reference Marotta, Santos, Cordeiro, Matos, Barros, Madeira, Bell-Sakyi and Fonseca2018) from small samples of two unrelated tick species removed from very different hosts (peccary and cattle) suggests that ticks in Brazil may frequently harbour trypanosomes. Further studies are likely to reveal even more novel species of this haemoparasite.
Description
Name: Trypanosoma amblyommi sp. nov.
Mammalian host: Unknown.
Location: Itatiaia National Park, Itatiaia, State of Rio de Janeiro, Brazil.
Vector: Possibly the tick A. brasiliense.
Biology and morphology
This species was isolated in co-cultivation with IDE8 tick cells and grown in L15B medium supplemented with FCS. The developmental stages found in axenic cultures were epimastigotes (predominantly), trypomastigotes and spheromastigotes. The mean total body length in the epimastigote form was 41.72 µ m, free flagellum 10.74 µ m and longitudinal axis of the kinetoplast 1.23 µ m. Measurement of total body length in the trypomastigote form was on average 35.81 µ m, free flagellum 10.76 µ m and longitudinal axis of the kinetoplast 1.09 µ m. The mean total body length in the spheromastigote form was 19.44 µ m, free flagellum 11.61 µ m and longitudinal axis of the kinetoplast was 1.05 µ m.
Molecular characteristics
The trypanosome presents amplified products for the 24Sα rDNA gene of about 270 bp using D75/D76 primers. In the first nested-PCR reaction for the 18S rDNA gene using the primers TRY927F and TRY927R, the amplified fragment was 900 bp. In the second reaction, using the SSU561F and SSU561R primers, the amplified fragment was 700 bp. In the phylogenetic analysis of ribosomal genes, this trypanosome is close to Trypanosoma sp. KG1 and T. caninum.
Storage
Axenic cultures of these trypanosomes are cryopreserved in 10% DMSO, stored in liquid nitrogen at −196 °C and deposited in the Parasitic Diseases Laboratory (LDP), located in Annex I of the Veterinary Institute, Department of Epidemiology and Public Health, Federal Rural University of Rio de Janeiro (UFRRJ), municipality of Seropedica, state of Rio de Janeiro, Brazil.
Acknowledgements
The authors thank Professor Ulrike Munderloh, University of Minnesota, and the Tick Cell Biobank for providing the tick cell line IDE8, and the Parque Nacional de Itatiaia, Municipio de Itatiaia, RJ, Brasil for providing the ticks used in the present study.
Financial support
This study was supported by grants from the Fundação de Amparo à Pesquisa do Rio de Janeiro (A. H. F., grant number E 26/201.144/2014) – (Research Support Foundation of the State of Rio de Janeiro – FAPERJ); Conselho Nacional de Desenvolvimento Científico e Tecnológico (National Council for Scientific and Technological Development – CNPq) (A. H. F., 305480/2013-8); and the UK Biotechnology and Biological Sciences Research Council (L. B. S., grant number BB/P024270/1).
Conflict of interest
None.






