INTRODUCTION
The nematode Aelurostrongylus abstrusus is a worldwide occurring cat lungworm with prevalences varying between 0.5 and 39.7% in Europe (Barutzki and Schaper, Reference Barutzki and Schaper2011; Knaus et al. Reference Knaus, Rapti, Shukullari, Kusi, Postoli, Xhaxhiu, Silaghi, Hamel, Visser, Winter and Rehbein2014), Australia (Palmer et al. Reference Palmer, Thompson, Traub, Rees and Robertson2008; Lacorcia et al. Reference Lacorcia, Gasser, Anderson and Beveridge2009), North and South America (Lucio-Forster and Bowman, Reference Lucio-Forster and Bowman2011; De Souza Ramos et al. Reference De Souza Ramos, Alves da Cruz Scheremeta, Soares de Oliveira, Sinkoc and de Campos Pacheco2013). Infected cats mostly show respiratory signs (Grandi et al. Reference Grandi, Calvi, Venco, Paratici, Genchi, Memmi and Kramer2005; Traversa et al. Reference Traversa, Di Cesare, Milillo, Iorio and Otranto2008), but non-pathognomic signs (Genchi et al. Reference Genchi, Ferrari, Fonti, De Francesco, Piazza and Viglietti2014; Schnyder et al. Reference Schnyder, Di Cesare, Basso, Guscetti, Riond, Glaus, Crisi and Deplazes2014) or asymptomatic cats are also commonly observed (Genchi et al. Reference Genchi, Ferrari, Fonti, De Francesco, Piazza and Viglietti2014). In some cases, an infection with A. abstrusus may result in death (Gerdin et al. Reference Gerdin, Slater, Makolinski, Looney, Appel, Martin and McDonough2011; Dirven et al. Reference Dirven, Szatmári, Van den Ingh and Nijsse2012; Philbey et al. Reference Philbey, Krause and Jefferies2014). Aelurostrongylus abstrusus has an indirect lifecycle in which gastropods serve as intermediate hosts (Hobmaier and Hobmaier, Reference Hobmaier and Hobmaier1935; Gerichter, Reference Gerichter1949) and various animals, such as rodents, reptiles and birds (Hamilton and McCaw, Reference Hamilton and McCaw1967; Scott, Reference Scott1973; Jezewski et al. Reference Jezewski, Bunkowska-Gawlik, Hildebrand, Perec-Matysiak and Laskowski2013) can serve as paratenic hosts. Since Hobmaier and Hobmaier's studies (Reference Hobmaier and Hobmaier1935), different molluscs have been used as intermediate hosts and the development of A. abstrusus from first (L1) to third stage (L3) larvae has been described (Gerichter, Reference Gerichter1949; Wallace and Rosen, Reference Wallace and Rosen1970; Ash, Reference Ash1970; Lopez et al. Reference Lopez, Panadero, Paz, Sanchez-Andrade, Diaz, Diez-Banos and Morrondo2005; Di Cesare et al. Reference Di Cesare, Crisi, Di Giulio, Veronesi, Frangipane di Regalbono, Talone and Traversa2013; Giannelli et al. Reference Giannelli, Ramos, Annoscia, Di Cesare, Colella, Brianti, Dantas-Torres, Mutafchiev and Otranto2014).
Biomphalaria glabrata is a neotropical, freshwater pulmonate snail. Because of its essential role as a natural intermediate host for Schistosoma mansoni, B. glabrata has been studied and maintained in laboratories for a long time. Consequently, suitable conditions for breeding and maintenance of this very rapidly reproducing snail are well known (DeJong et al. Reference DeJong, Morgan, Paraense, Pointier, Amarista, Ayeh-Kumi, Babiker, Barbosa, Brémond and Canese2001; Pointier et al. Reference Pointier, David and Jarne2005). B. glabrata has previously been used as an intermediate host for A. abstrusus (Ash, Reference Ash1970; Wallace and Rosen, Reference Wallace and Rosen1970; Jefferies et al. Reference Jefferies, Vrhovec, Wallner and Catalan2010). However, descriptions of the chronological and morphometric development of A. abstrusus L1 to L3 in this snail and the success rate of snail infections, which are key factors for the identification and recovery of infective larval stage for further experimental studies, were missing to date.
The aims of this study were to describe and assess the development rate from L1 to the infective and relevant L3 stage of A. abstrusus in the snail B. glabrata, as well as to evaluate the suitability of this species as an experimental intermediate host.
MATERIAL AND METHODS
The A. abstrusus L1 used for the infection of 306 B. glabrata snails (sized 0.8–1.5 cm in diameter) were obtained from feces from a 2 to 3 months old naturally infected female stray cat with high-grade dyspnoea presented at the Animal Hospital of the Vetsuisse Faculty of the University of Zurich. L1 were isolated using the Baermann–Wetzel technique (Deplazes et al. Reference Deplazes, Eckert, Von Samson-Himmelstjerna, Zahner, Deplazes and Eckert2013) and analysed with a duplex-polymerase chain reaction (PCR) (Annoscia et al. Reference Annoscia, Latrofa, Campbell, Giannelli, Ramos, Dantas-Torres, Brianti and Otranto2014) to verify their species identity and to exclude a co-infection with the lungworm Troglostrongylus brevior.
The snails were bred at the Institute of Parasitology at the Vetsuisse Faculty of the University of Zurich and thus, had no previous contact with other parasites. Prior to infection, the snails were fasted for 24 h in order to increase the ingestion rate, and placed individually into the wells of a 24 well polystyrene cell-culture plate (Nunc™, Roskilde, Denmark) with 2–3 mL of tank water, each containing 300 L1 of A. abstrusus. After 24 h the wells were checked for remaining L1 with a stereomicroscope (Leica® MS 5, Leica Microsystems GmbH, Wetzlar, Germany) and the snails placed into two large tanks. They were maintained under standardized conditions at constant temperature (24–26 °C) and fed on lettuce, in accordance with the accepted principles of animal welfare for invertebrates. Every week, five snails were randomly chosen, humanely euthanized by prompt crushing and digested individually for 1 h in 50 mL H2O, 1% HCl containing 0.12 g Pepsin (800–2500 U mg−1, Sigma P700, Sigma-Aldrich, Missouri, USA) in a water bath rotary shaker (Aquatron®, Infors AG, Basel, Switzerland) at 45 °C. After digestion, the tubes containing the material were centrifuged at 500 × g for 3 min. Each pellet was re-suspended in tap water, centrifuged again and the sediment examined under the stereomicroscope. Larvae were stained with Lugol, photographed, measured by using a digital image processing system (Leica® DM 100 LED, Leica® DFC 420, Leica® LAS 4, Leica Microsystems GmbH, Wetzlar, Germany) and morphologically identified based on previously described developmental stage characteristics (Gerichter, Reference Gerichter1949; Di Cesare et al. Reference Di Cesare, Crisi, Di Giulio, Veronesi, Frangipane di Regalbono, Talone and Traversa2013). Starting from the day when all isolated larvae had developed into L3, the time interval between digestions was extended to every second week until the end of the trial (28 weeks post exposure, wpe). In the eighth and ninth wpe, 200 snails were removed to extract the L3 for other purposes.
Microsoft Excel 2010 for Windows (Microsoft Corporation, Redmond, USA) was used to calculate means and standard deviations (SD). Statistical analysis was performed using Windows IBM® SPSS ® Statistics (Version 22). The Mann–Whitney U-Test was used for comparing the number of larvae per snail across time points. Differences with P < 0.05 were considered statistically significant.
RESULTS
As confirmed by PCR, A. abstrusus was the only parasite present in the cat feces from which L1 were isolated. Twenty-four hours after snail exposure to L1, a mean of 10.1 L1 (SD: 14.02 L1) per well were counted, indicating a penetration rate of 96.6%. Morphological features are presented in Fig. 1A–C. L1 presented the typical notched S-shaped tail with a dorsal dent, L2 displayed a characteristic outer sheath, and L3 showed a round knob at the tip of the tail.
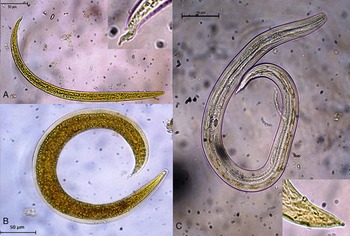
Fig. 1. Aelurostrongylus abstrusus larvae: (A) first-stage larva and detail of the tail end; (B) second-stage larva; (C) third-stage larva and detail of the tail end (stained with Lugol).
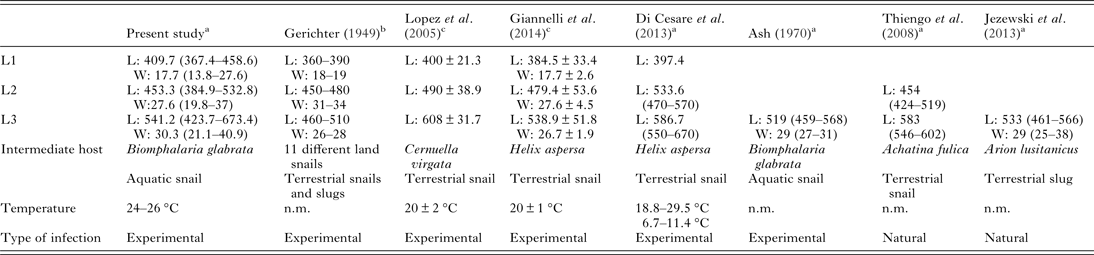
L1 were present until 6 wpe. In the first wpe a high number of L1 in the snails were damaged. The first L2 were obtained in the second wpe, while the first L3 occurred in the fourth wpe. After 8 wpe, all larvae had reached the L3 stage (Fig. 2). Except for one dead L3 observed after artificial digestion, all recovered L3 were alive and motile throughout the experiment. Larval sizes (length and width) are summarized in Table 1 and are presented with results from previously published studies. L3 were isolated from snails until 26 wpe, while at 28 wpe, the remaining last five snails were all found exempt of L3. Development times for the different larval stages of A. abstrusus are shown in Table 2, besides findings from other studies where different snail species were used.

Fig. 2. Occurrence of first (L1), second (L2) and third-stage larvae (L3) of Aelurostrongylus abstrusus in Biomphalaria glabrata snails in weeks post exposure (=wpe).
Table 1. Morphometric data of the developmental stages (first stage (L1), second stage (L2) and third stage (L3)) of Aelurostrongylus abstrusus larvae in intermediate hosts obtained from the present work and from reports published elsewhere (measurements in μm: L: body length, W: body width). n.m. = not mentioned
a Mean and range in brackets.
b Range.
c Mean and standard deviation.
Table 2. Key time points in the development from first- to third-stage Aelurostrongylus abstrusus larvae in different gastropods in days post exposure (dpe) from the present work and from previous reports

S, summer; W, winter; n.e., not evaluated.
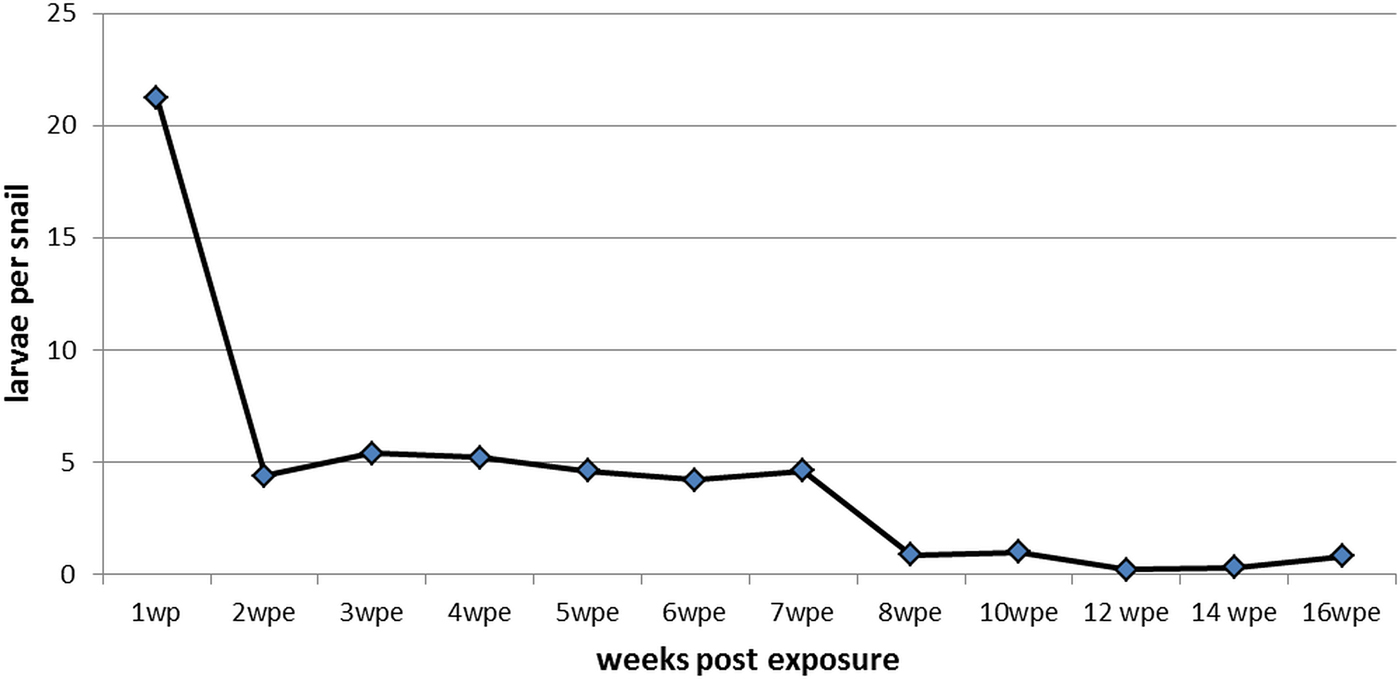
From the first wpe (mean: 21.2, SD: 14.3) to the second wpe (mean: 4.4, SD: 8.3), we observed a significant reduction of larvae per snail (P < 0.05). Afterwards, the number of larvae per snail remained constant. From the second to the seventh wpe, a mean of 4.7 (SD: 0.5) larvae per snail were recovered (Fig. 3). After 8 wpe, a further non-significant decrease in the number of recovered larvae was noted, down to a mean of 0.6 (SD: 0.3; P > 0.05) larvae per snail. In total, only 0.4% of the L1 used for inoculation of the snails completely developed into L3 in the course of the study.
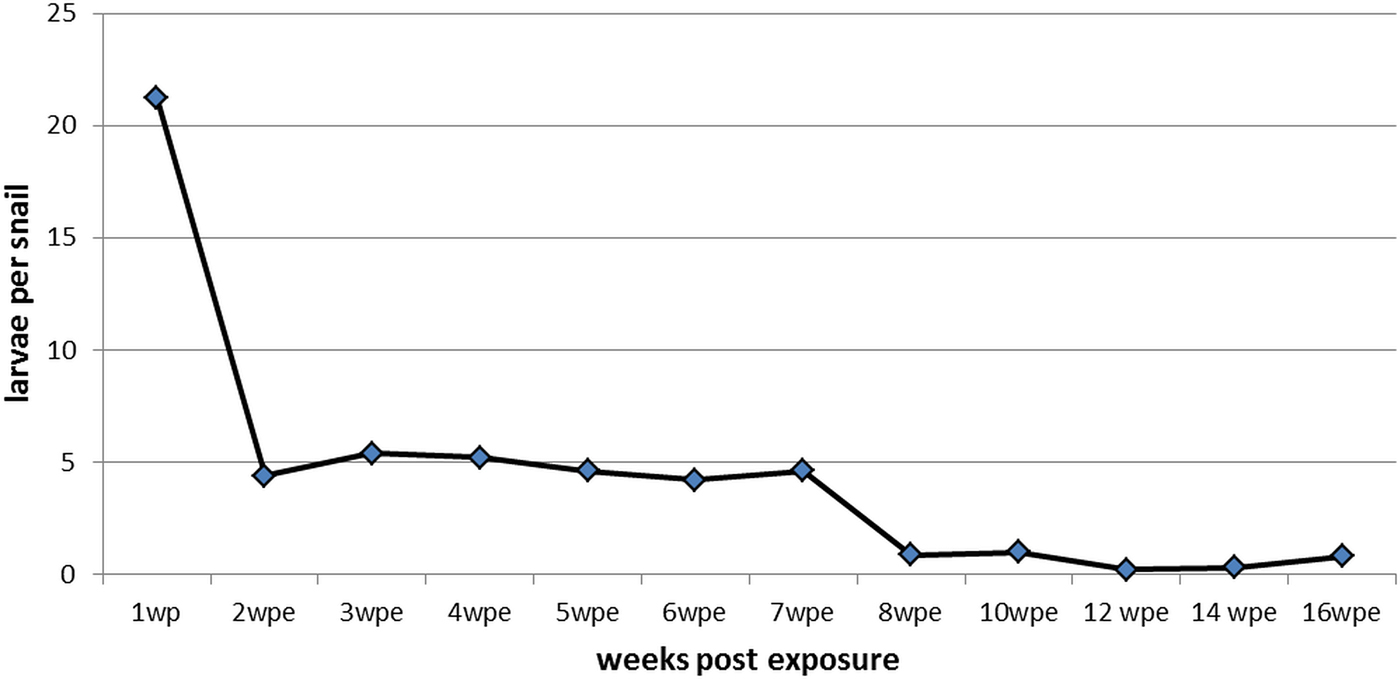
Fig. 3. Mean number of Aelurostrongylus abstrusus larvae (first, second and third stage) per dissected Biomphalaria glabrata snail over 16 weeks post exposure (wpe).
DISCUSSION
Our data confirm the feasibility of experimental infections with the tropical freshwater snail B. glabrata as a potential intermediate host for the felid lungworm A. abstrusus. However, there are indications that this non-terrestrial snail species is not an adequate intermediate host for mass production of A. abstrusus L3.
Our measurements of total length and width of L1, L2 and L3 are in line with previously published numbers (Table 1). However, we obtained a broader range of length and width within each developmental stage than what others reported, while in our hands, L3 tended to be longer in size than previously described for L3 recovered from the same snail species (Ash, Reference Ash1970). As suggested elsewhere, the size of snails as well as the duration of the life cycle within the gastropods may influence the size of the larvae (Di Cesare et al. Reference Di Cesare, Crisi, Bartolini, Iorio, Talone, Filippi and Traversa2015). Furthermore, also the fixative and brightening agents can influence the measured dimensions of larvae (Lopez et al. Reference Lopez, Panadero, Paz, Sanchez-Andrade, Diaz, Diez-Banos and Morrondo2005).
Comparing the larval development time to the L3 stage with prior studies (Table 2), the occurrence of L3 appears delayed in the present work (4 wpe) than in investigations carried out with the terrestrial snails Helix aspersa (15 and 21 dpi) and Cernuella virgata (18 dpi). Nonetheless, the time point when all larvae had reached the L3 stage (8 wpe) was consistent with the findings of the other authors.
As many as 96.6% of L1 had invaded the snails after 24 h, representing a higher penetration rate than described by Lopez et al. (Reference Lopez, Panadero, Paz, Sanchez-Andrade, Diaz, Diez-Banos and Morrondo2005), with 65.5% in experimentally infected C. virgata. The mechanisms for infecting aquatic and terrestrial snails are different, which may explain discrepancies in terms of penetration and development rate. Nematodes infect aquatic snails more often passively, through accidental ingestion by the snail, and less commonly by direct penetration of the snail tegument. In contrast, in terrestrial snails direct penetration, mostly through the foot, is a very common mechanism of infection (Morley, Reference Morley2010). In the study by Lopez et al. (Reference Lopez, Panadero, Paz, Sanchez-Andrade, Diaz, Diez-Banos and Morrondo2005) nearly 5% of the L1 developed into L3. Di Cesare et al. (Reference Di Cesare, Crisi, Di Giulio, Veronesi, Frangipane di Regalbono, Talone and Traversa2013) obtained an even higher development rate, with up to 50% developing in H. aspersa. In the present study the percentage of larvae completing their development into L3 was limited to 0.4%. Therefore, a high ingestion/penetration rate is not necessarily an indication for a high development rate. The reasons behind this low development rate in the present study remain unclear. The snail species, size and age, the L1 isolate, its age and the infective dose, as well as the environmental conditions may all influence the development rate (Di Cesare et al. Reference Di Cesare, Crisi, Bartolini, Iorio, Talone, Filippi and Traversa2015). Environmental conditions were described to have an important impact on larval development in intermediate hosts: the maturation is faster and more larvae start and complete their development at higher temperatures, respectively, in summer, i.e. in H. aspersa snails infected with A. abstrusus (Di Cesare et al. Reference Di Cesare, Crisi, Di Giulio, Veronesi, Frangipane di Regalbono, Talone and Traversa2013; Giannelli et al. Reference Giannelli, Ramos, Annoscia, Di Cesare, Colella, Brianti, Dantas-Torres, Mutafchiev and Otranto2014). As we kept the environmental factors constant at the indicated temperatures for B. glabrata under established experimental conditions, other reasons for this low development rate have to be considered. In the past, we used to have a higher development rate of A. abstrusus L3 in B. glabrata at our institute (data not shown). Since the L1 originated from different cats, a diverging infectivity of the isolate cannot be excluded. Concerning the infective dose per snail, Wallace and Rosen (Reference Wallace and Rosen1970) infected B. glabrata with 100–800 A. abstrusus L1, obtaining a higher development rate. Since we had minor loss of snails over 28 wpe (n = 6), we conclude that the number of L1 (n = 300) used in our study should have been well tolerated by the snails.
Previous studies showed that after snails are infected with nematode larvae, their defence mechanisms are activated (Sauerländer, Reference Sauerländer1976; Pereira et al. Reference Pereira, Martins-Souza, Coelho, Lima and Negrao-Correa2006; Barcante et al. Reference Barcante, Barcante, Fujiwara and Lima2012). The difference between i.e. a penetration rate of 65.5% and a development rate of 9.96% into later stages suggested that a majority of L1 are destroyed during or shortly after penetration (López et al. Reference Lopez, Panadero, Paz, Sanchez-Andrade, Diaz, Diez-Banos and Morrondo2005). In the present study we observed a significant drop of L1 per snail from the first to the second wpe. Furthermore, in the first wpe a high number of L1 in the snails were damaged. This strengthens the concept that a high number of incorporated larvae may not start their development and are destroyed by the immune system of the snails. Other reasons for the reduction of larval numbers within snails over time are possible. The last A. abstrusus positive snails in this study were identified at 26 wpe, while it has been shown in H. aspersa that snails can remain infected for up to 2 years (Hamilton, Reference Hamilton1969). This might additionally suggest that in the most suitable intermediate hosts larvae may survive as long as the snails live. On the other hand, we cannot rule out the possibility that the absence of larvae at 28 wpe in B. glabrata was due to spontaneous departure of L3 from the snails, as it has been demonstrated for the same snail species infected with Angiostrongylus vasorum, another metastrongyloid nematode (Barcante et al. Reference Barcante, Barcante, Dias and Lima2003), or in H. aspersa infected with A. abstrusus (Giannelli et al. Reference Giannelli, Colella, Abramo, do Nascimento Ramos, Falsone, Brianti, Varcasia, Dantas-Torres, Knaus, Fox and Otranto2015).
Due to its high fertility and easy maintenance, Biomphalaria glabrata is not only a naturally occurring intermediate host but also a perfect experimental intermediate host for S. mansoni (DeJong et al. Reference DeJong, Morgan, Paraense, Pointier, Amarista, Ayeh-Kumi, Babiker, Barbosa, Brémond and Canese2001; Pointier et al. Reference Pointier, David and Jarne2005), and proved its suitability for experimental infections also with other metastrongylid parasites such as A. vasorum (Ash, Reference Ash1970; Barcante et al. Reference Barcante, Barcante, Fujiwara and Lima2012). The findings presented here describe in detail the development of A. abstrusus from L1 to L3 and the development rate in B. glabrata. In particular, this study provides new insights into the chronological development of A. abstrusus in the aquatic snail. Knowledge of the time needed for all larvae to reach the infectious L3 stage and awareness of the potentially very low development rate are critical for the planning of subsequent experimental studies with metastrongyloid parasites.
ACKNOWLEDGEMENTS
The authors would like to thank Stefan Müller and Annakatrin Häni for their support regarding maintenance of the infected snails, Dr Felix Grimm for his help with the biomolecular methods and Dr Lucienne Tritten for critical review of the manuscript. This publication is part of the doctoral thesis of Eva-Maria Zottler.
FINANCIAL SUPPORT
We thank Bayer HealthCare Animal Health for financial support of the doctoral thesis of Eva-Maria Zottler.
CONFLICT OF INTEREST
None.
ETHICAL STANDARDS
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional guides on the use of invertebrate animals.