Introduction
Patients with cancer are at risk of distress, which is defined by the National Comprehensive Cancer Network (NCCN) as a multifaceted unpleasant experience that ranges across the spectrum of feelings of sadness to psychological conditions such as depression and anxiety that can affect a person’s capacity to cope with the illness (Riba et al. Reference Riba, Donovan and Andersen2019). This is because they face an array of problems and often have unmet health needs from various domains, including physical, psychological, and social across their cancer trajectory (Donovan et al. Reference Donovan, Grassi and Deshields2020). The reported prevalence of cancer-related distress is significant, ranging from 22 to 52% in studies across major cancer types in the United States (Zabora et al. Reference Zabora, BrintzenhofeSzoc and Curbow2001), Taiwan (Wang et al. Reference Wang, Cheng and Feng2017), and Germany (Mehnert et al. Reference Mehnert, Hartung and Friedrich2018). Cancer-related distress is associated with poorer health outcomes such as treatment nonadherence (DiMatteo et al. Reference DiMatteo, Lepper and Croghan2000; Riba et al. Reference Riba, Donovan and Andersen2019), reduced quality of life (Ehlers et al. Reference Ehlers, Davis and Bluethmann2018; Head et al. Reference Head, Schapmire and Keeney2012; Riba et al. Reference Riba, Donovan and Andersen2019), and possibly greater mortality (Barry et al. Reference Barry, Stout and Lynch2020; Batty et al. Reference Batty, Russ and Stamatakis2017).
Breast and gynecological cancers are common in Singapore (National Registry of Disease Office, Health Promotion Board 2021). With an aging population and higher survival rates from earlier detection and improved treatment, there would be an increasing number of cancer survivors with more care needs and consequently distress. Understanding factors associated with distress may help to identify patients with higher care needs, so that finite health-care resources could be directed to them for better utilization. Factors associated with distress have been explored in the literature, and possible factors include younger age, preexisting comorbidities, history of mental health issues, advanced cancer diagnosis, and functioning limitations (Riba et al. Reference Riba, Donovan and Andersen2019; Syrowatka et al. Reference Syrowatka, Motulsky and Kurteva2017).
Patients with cancer-related distress may have different health-care service utilization patterns, and a better understanding of this may be helpful to guide health-care delivery in the long run (Meyers Reference Meyers and Boslaugh2008). However, the current evidence on the association between distress and health-care service utilization is inconclusive. A study in the United States on 4326 breast, prostate, and colorectal cancer patients across survivorship stages found that patients with serious psychological distress had higher utilization of emergency department (ED), inpatient and outpatient services, and total medical expenditures compared to patients without (Han et al. Reference Han, Lin and Li2015). Another study that used Distress Thermometer and Problem List (DTPL) for distress screening in 848 patients with metastatic lung and non-colorectal gastrointestinal cancer on active treatment found that distress was associated with increased odds of hospitalization or ED visit, within 3 and 6 months (Hildenbrand et al. Reference Hildenbrand, Park and Casarett2020). However, in a smaller study of 245 mixed cancer survivors in the Netherlands using the Hospital Anxiety and Depression Scale, there was a lack of association between psychological distress and acute health-care utilization (Compen et al. Reference Compen, Adang and Bisseling2018). While the available literature seems to suggest a possible positive association between distress and acute health-care service utilization in oncology, there is merit to see if a positive association is consistently replicated in a different setting, particularly in the Asian setting where data are scarce.
As such, the aims of this study were as follows: first, to identify demographic and clinical factors associated with distress in a population of breast and gynecological cancer patients across survivorship stages; second, to assess if self-reported high distress was associated with subsequent acute health-care service utilization in terms of hospital admissions and ED visits, and we hypothesized that cancer-related distress would be associated with greater acute health-care service utilization.
Methods
Study design and population
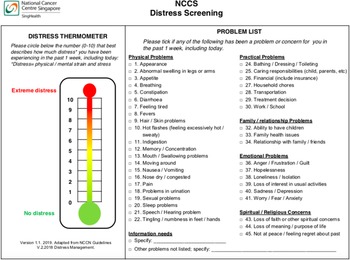
This was a retrospective study of breast and gynecological cancer patients who had a medical oncologist outpatient visit at the National Cancer Centre Singapore from 16 September 2019 to 31 July 2020. It is the largest ambulatory cancer center in Singapore and treats almost 70% of the cancer patients in Singapore’s public health-care sector. Inclusion criteria included the following: (a) age ≥ 21 years, (b) a confirmed diagnosis of breast or gynecological cancers, and (c) completed their distress screening with DTPL (Figure 1) before the scheduled clinical visit as part of an ongoing service development project for early supportive care. This study was approved by the SingHealth Centralised Institutional Review Board: 2020/2789.

Fig. 1. National Cancer Centre Singapore (NCCS) distress screening.
Data collection and procedure
Data on patients’ demographics, clinical characteristics, and acute health-care service utilization were extracted from institutional electronic medical records, merged using unique identifiers for each patient, and anonymized for analysis.
Patients completed the DTPL either via an online platform or face to face in a clinic with a trained research personnel prior to the oncologist appointment. Only the first distress score for each patient within the study period was included for analysis.
Measures
Demographics and clinical information
Demographic variables included age, nationality, gender, ethnicity, marital status, paying class, and employment. Clinical data included cancer diagnosis, number of malignancies, presence of metastasis, time since cancer diagnosis, Eastern Cooperative Oncology Group (ECOG) performance status, comorbidities, and presence of psychiatric diagnosis. The variable “payment class,” which differentiates between paying and subsidized patients, was used as a proxy for social economic status. If a patient had more than one cancer diagnosis, the most recent diagnosis was selected as this would be more relevant to the reported distress level and outcome measures. The duration of time from cancer diagnosis to distress screening was derived as the difference between the diagnosis date and the distress screening date. We categorized the time from cancer diagnosis into 3 cancer survivorship phases (Miller et al. Reference Miller, Merry and Miller2008) with clinically relevant cutoffs: (1) 0–1 year accounts for an active treatment phase, (2) >1 to 5 years is post initial treatment, with any ongoing long-term therapy to reduce recurrence and close surveillance phase, and (3) >5 years represents the cessation of treatment or remission phase. The burden of comorbidity was assessed based on the Charlson Comorbidity Index but excluding cancer variables and unadjusted for age, using the past medical history information collected.
Distress thermometer and problem list
The distress thermometer (DT) has been locally validated in a mixed cancer population (Lim et al. Reference Lim, Mahendran and Chua2014) and consists of a 11-point analog scale from 0 (“No distress”) to 10 (“Extreme distress”), where patients self-report their distress level in the past week. The problem list (PL) has been locally adapted with face validity by a group of health-care professionals and contains a problem checklist with 5 categories: physical problems (22 items), practical problems (7 items), family/relationship problems (3 items), emotional problems (6 items), and spiritual/religious concerns (3 items) where patients identify the problems they had in the past week (Figure 1). The locally adapted DTPL has also been translated to other languages: Chinese, Malay, and Tamil accordingly by the same team. A cutoff ≥4 was used to identify patients with high distress as per NCCN recommendations (Riba et al. Reference Riba, Donovan and Andersen2019), and this was also supported by a meta-analysis in Asian patients (Sun et al. Reference Sun, Thapa and Wang2020).
Acute health-care service utilization
Acute health-care service utilization was defined by whether the patient had any documented (1) ED visit or (2) hospitalization in Singapore General Hospital within 30 days after the distress screening. Singapore General Hospital is the largest tertiary hospital in Singapore and the primary hospital associated with National Cancer Centre Singapore. A shorter 30-day follow-up period was chosen to minimize possible confounding factors (e.g., other intervening events) from influencing outcome measures. All-cause ED visits and hospitalizations were included.
Statistical analysis
Descriptive statistics were reported in counts and percentages for categorical variables, mean and standard deviation (SD) or median and interquartile range (IQR) for normally and non-normally distributed continuous variables, respectively. Pearson’s Chi-square tests were used for categorical variables, independent t-test, and Mann–Whitney U test for normally and non-normally distributed continuous variables, respectively.
For the evaluation of factors associated with distress, a forward stepwise variable selection approach with logistic regression was used to identify demographic and clinical variables that were associated with high distress, with entry p-value = 0.1 and exit p-value = 0.105. Univariable and multivariable logistic models provided crude and adjusted odds ratios (OR) with 95% confidence interval (CI). As patients in different survivorship phases (i.e., time from cancer diagnosis) might have differences in their health condition, subgroup analysis was performed for factors in the main analysis that were found to be associated with high distress.
To evaluate the associations between high distress and ED visits as well as hospitalizations, logistic regression was used with adjustments made for covariates. The variable list was derived from a literature search on variables that are associated with ED visits or hospitalizations (Lash et al. Reference Lash, Bell and Reed2017; Prince et al. Reference Prince, Powis and Zer2019). Univariate analysis was conducted to determine the relationship between demographic/clinical variables and distress levels as well as outcome variables (ED visits/hospitalizations). Variables were determined to be (1) potential confounders if they were found to be statistically significantly associated with both distress levels and ED visits/hospitalizations or (2) clinically relevant variables if they were only associated with the outcome variables. In the model building, after specifying distress as a priori, relevant variables were added individually and compared against nested models, evaluated with Akaike information criteria and Bayesian information criteria for model fitting. Two best-fit models were built for each outcome measure (ED visits/hospitalizations), with the first model adjusting for potential confounders only and the second one adjusting for additional clinically relevant variables. To test whether there were differences in the impact of distress on ED visits or hospitalizations among the 3 different survivorship phases (i.e., time from cancer diagnosis), possible interaction effects between distress and survivorship phases were explored.
Two-sided statistical tests were performed, and a p-value of <0.05 denotes statistical significance. All statistical analyses were conducted with Stata/SE 16.1.
Results
Cohort characteristics
Among 1455 patients who completed distress screening between 16 September 2019 and 31 July 2020, 1386 patients were included in the analysis. The flow diagram and reasons for exclusion are summarized in Figure 2. Overall, the mean age was 59.4 ± 11.1 years and 1382 patients (99.7%) were female because of the cancers of interest: 1238 patients (89.3%) had breast cancer while 52 patients (10.7%) had gynecological cancer as their latest cancer diagnosis.

Fig. 2. Flow diagram for selection of patients included for final analysis.
Based on the DT cutoff score of 4, 510 patients (36.8%) were classified as having high distress. The demographics and clinical characteristics of the patients are summarized in Table 1. Apart from age, time from diagnosis, ECOG performance status, and presence of psychiatric diagnosis, both groups were comparable in other demographics and clinical characteristics.
Table 1. Baseline demographics and clinical characteristics of patients in low and high distress group

Note: ECOG, Eastern Cooperative Oncology Group; IQR, interquartile range; and SD, standard deviation.
* p < 0.05, statistically significant difference.
a Ethnicity (others): Bangladeshi, Burmese, Caucasian, Eurasian, Filipino, Indonesian, Japanese, Pakistani, Sikh, Thai, and Vietnamese.
b Anxiety, depression, polysubstance abuse/dependence, obsessive compulsive disorder, schizophrenia, mood disorder, adjustment disorder, bipolar disorder, acute stress reaction, and panic disorder.
Factors associated with distress
Factors associated with high distress are found in Table 2. For demographic variables, only age was associated with self-reported high distress. Compared to patients 40–65 years old, patients <40 years old had a 1.48 (95% CI 0.83–2.63) higher odds of distress and those ≥65 years old had a 0.58 (95% CI 0.44–0.77) lower odds of distress. For clinical variables, presence of psychiatric disorder, ECOG performance status, and time from cancer diagnosis were associated with distress. Compared to those without psychiatric diagnosis, patients with psychiatric diagnosis had a 2.53 (95% CI 1.52–4.21) higher odds of distress. Compared to those with ECOG 0, patients with ECOG 1 had a 1.46 (95% CI 1.07–2.00) higher odds of distress and those with ECOG ≥ 2 had 2.93 (95% CI 1.69–5.06) higher odds of distress. Compared to patients who had cancer diagnosis for >1 to 5 years, patients who had cancer diagnosis for >0 to 1 year had a 1.42 (95% CI 1.06–1.89) higher odds of distress, while those with >5 years had a 0.998 (95% CI 0.75–1.33) lower odds of distress.
Table 2. Multivariable analysis of variables associated with high distress (predictors of distress)

Note: CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; OR, odds ratio; and Ref, reference.
a Adjusted for age, time from cancer diagnosis, presence of psychiatric diagnosis, and ECOG score.
The subgroup analysis found that among the factors identified to be associated with distress in the cohort, there were differences in the factors associated with distress among the 3 different survivorship phases (Supplementary Table S5). In the 0–1 year survivorship phase, only age was found to be associated with distress. In the >1 to 5 years survivorship phase, presence of psychiatric disorder and ECOG performance were found to be associated with distress. For >5 years survivorship phase, age, presence of psychiatric disorder, and ECOG performance status were all associated with distress.
Acute health-care service utilization
Within 30 days after distress screening, 38 (2.7%) patients had ≥1 ED visits (range: 1–3). Demographics and clinical characteristics of patients with and without ED visit(s) are summarized in Supplementary Table S1. Higher proportion of patients with high distress had ≥1 ED visits within 30 days after distress screening, compared to the low distress group (4.5% vs 1.7%). From the univariate analysis, the high distress patients had 2.71 (95% CI 1.40–5.24) times greater odds of having ≥1 ED visits within 30 days after distress screening than low distress patients. The association remains significant with OR 2.33 (95% CI 1.19–4.55) after controlling for potential confounders (i.e., time from cancer diagnosis and ECOG performance status), and OR was lower at 2.25 (95% CI 1.14–4.43) after controlling for additional predictors of ED visits (i.e., cancer diagnosis and age) (Table 3, Supplementary Table S3).
Table 3. Odds ratio for the association between distress levels and outcome measures (emergency department visits and hospitalization) within 30 days after self-reported Distress Thermometer and Problem List
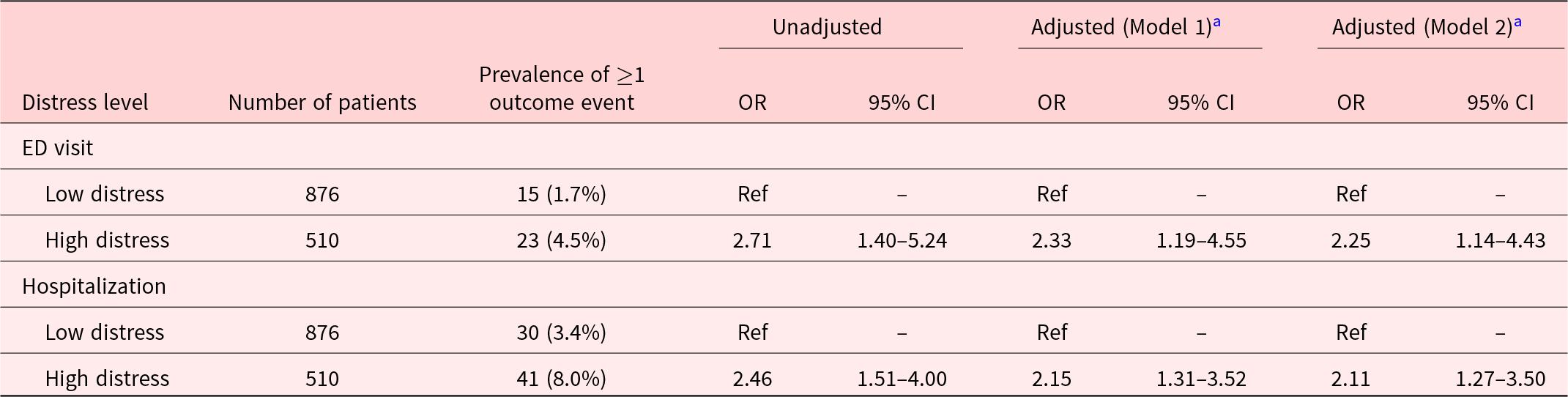
Notes: Model 1: adjusted for time from cancer diagnosis and ECOG performance status. Model 2: adjusted for time from cancer diagnosis, ECOG performance status, cancer diagnosis, and age. CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; and OR, odds ratio.
a n = 5 with missing ECOG performance status; hence, adjusted multivariate model is based on n = 1381.
Seventy-one patients (5.1%) had ≥1 all-cause hospitalization (range: 1–5) within 30 days after their distress screening. Demographics and clinical characteristics of patients with and without hospitalization(s) are summarized in Supplementary Table S2. Higher proportion of 8% in the high distress group had ≥1 hospitalization compared to 3.4% in the low distress group. Patients with high distress had 2.47 (95% CI 1.52–4.00) times greater odds of having ≥1 hospitalization within 30 days after distress screening compared to low distress patients. The association remained significant with OR 2.15 (95% CI 1.31–3.52) after controlling for potential confounders (i.e., time from cancer diagnosis and ECOG performance status) and 2.11 (95% CI 1.27–3.50) after controlling for both potential confounders and predictors of hospitalizations (i.e., cancer diagnosis and age) (Table 3, Supplementary Table S4). In addition, interaction effects between distress and survivorship phases (i.e., time from cancer diagnosis) were not found to be significant in both models (Supplementary Table S6).
Problem list
The high distress group identified significantly more problems, with a median of 5 problems (IQR: 2–8), compared to 1 problem (IQR: 0–3) (p < 0.0001) in the low distress group (Table 4). The prevalence of having ≥1 problem in each of the 5 domains were significantly greater in the high distress group: physical (88.8% vs 62.4%), emotional (62.0% vs 19.3%), practical (44.7% vs 19.2%), family/relationship (16.7% vs 4.3%), and spiritual/religion (13.1% vs 2.3%) (Table 4). The profile of problems in both groups also differed – the top 5 problems for the high distress group were feeling tired (47.5%), worry/fear/anxiety (45.7%), pain (37.1%), sleep problems (33.9%), and tingling/numbness in feet/hands (31.2%), while they were tingling/numbness in feet/hands (21.2%), feeling tired (20.4%), pain (17.7%), hair/skin problems (15.2%), and sleep problems (15.0%) for the low distress group (Table 5). Of note, most of the problems were physical in nature, apart from worry/fear/anxiety being an emotional problem, which was the second most common problem in the high distress group but the sixth most common problem in the low distress group (13.2%) (Table 5).
Table 4. Prevalence and median number of self-reported problems in total and across five domains: physical, emotional, practical, family/relationship, and spiritual

Note: Median with IQR only shown for domains with overall ≥25% prevalence. IQR, interquartile range.
* p < 0.05, statistically significant difference.
Table 5. Top 5 problems (in descending order) for patients in low and high distress group

Discussion
This study identified characteristics associated with high distress and explored the relationship between distress and acute health-care services utilization in a cohort of patients with breast and gynecological cancer across survivorship phases and cancer stages at an Asian cancer center. One out of 3 patients self-reported to have high distress on their first distress screening, with patients of younger age and having previous psychiatric diagnosis, poorer ECOG performance status, and shorter duration from cancer diagnosis being associated with high distress. Self-reported high distress using DTPL was associated with higher odds of having at least 1 ED visit or at least 1 hospitalization within 30 days post distress screening.
The 4 factors associated with distress in our cohort of breast and gynecological patients (i.e., younger age, previous psychiatric diagnosis, poorer ECOG performance status, and shorter duration from cancer diagnosis to distress screening) were broadly consistent with existing literature (Jewett et al. Reference Jewett, Teoh and Petzel2020; Jørgensen et al. Reference Jørgensen, Laursen and Garne2016; Riba et al. Reference Riba, Donovan and Andersen2019; Syrowatka et al. Reference Syrowatka, Motulsky and Kurteva2017). Even though NCCN guidelines (Riba et al. Reference Riba, Donovan and Andersen2019) identify presence of metastasis (proxy for advanced cancer diagnosis) and comorbidities as factors associated with distress, results from published literature were mixed. In a systematic review for breast cancer patient survivors (Syrowatka et al. Reference Syrowatka, Motulsky and Kurteva2017), only 3 out of the 21 included studies found an association for advanced cancer diagnosis, while 5 out of the 9 included studies found an association for comorbidities. Our study found that both the variables presence of metastasis and comorbidities were not associated with distress. A possible reason for lack of associations could be that patients with advanced cancer or patients with more comorbidities could have received more support in terms of health-care services, for example, palliative care, which could have allayed their problems and hence their sources of distress. The discussion of the subgroup analysis of factors associated with distress among different survivorship phases (i.e., time from cancer diagnosis) is beyond the scope of this paper, given the exploratory intent to identify factors of distress with the cohort, and would be a further area of research in future papers.
Developing a better understanding of the clinical and demographic profiles associated with high distress may facilitate more efficient allocation of resources for earlier intervention in patients and in turn reduce future unnecessary acute health-care utilization. This information could also guide the development of processes to proactively screen for distress in at-risk population. Rather than providing supportive services for all patients with cancer, some of whom may not require them, identifying a subpopulation with clinical or demographic factors associated with high distress could facilitate more efficient resource allocation to patients who are more likely to require supportive care.
Current evidence regarding the impact of distress on acute health-care services utilization is limited and inconclusive, particularly for the Asian setting. Our study found that self-reported high distress was associated with higher odds of acute health-care service utilization. This was similar to the trend based on available literature, even though direct comparisons with studies (Han et al. Reference Han, Lin and Li2015; Hildenbrand et al. Reference Hildenbrand, Park and Casarett2020) that showed positive associations would be limited by the heterogeneity in study designs, measures used, and cancer populations. Underlying mechanisms behind the association between self-reported distress and ED visits or hospitalizations have not been clearly elucidated in the literature. These can be multifactorial and possibly changing along the patients’ cancer trajectory – for instance patients in the active treatment phase may encounter more physical problems related to treatment side effects, while long-term survivors may be more worried about recurrence. This complexity is further compounded by the broad construct of cancer-related distress, which encompasses various domains – psychological, physical, social, and spiritual (Riba et al. Reference Riba, Donovan and Andersen2019).
One possible suggestion for the association between distress and acute health-care service utilization could possibly be mediated by symptom perception (i.e., individuals’ complaints about physical symptoms such as fatigue or chest symptoms) which is related to poor perceived health (Koopmans and Lamers Reference Koopmans and Lamers2007). Distress could also be a proxy of the symptom burden that is associated with ED visits and hospitalizations. In this study, we found that patients with self-reported high distress had identified significantly more problems compared to those with low distress. In particular for the top 5 problems, there were significantly higher prevalence of physical (fatigue, pain, sleep issues, and peripheral neuropathy) and emotional (worry/fear/anxiety) problems in the high distress group. In addition, given that the problem of worry/fear/anxiety was significantly higher in the high distress group, it can be postulated that the psychological element of distress could result in reduced coping mechanisms and reduced threshold for seeking tertiary health-care services. Future studies could explore the possible underlying mechanisms that could account for the impact of distress on utilization of acute care services.
This study had a few limitations. First, health-care services utilization data from only one hospital was available and this could underestimate the true utilization frequency. However, most admissions should be captured as most patients would be admitted to this hospital, which is colocated within the same campus as the outpatient cancer service. Second, due to the retrospective nature of the study, data collected was limited to variables routinely collected in the electronic medical records and some data could not be accurately extracted from the clinical records for meaningful analysis. For instance, details on cancer staging were not updated and the end dates of treatment, for example, hormonal therapy, chemotherapy, were not available to identify if patients were still on treatment, and arbitrary assumptions to account for them could introduce more confounders. Therefore, to mitigate these issues, the stage of cancer was accounted for by the presence of metastasis as that has the most significant impact on the prognosis and treatment intent, while time from cancer diagnosis to distress screening was used as a proxy for how likely patients would be on active treatment. The variable “payment class” was used as a proxy for social economic status, which was also not available.
In conclusion, this study identified factors associated with distress in breast and gynecological cancer patients in Singapore, which would be helpful with identification of subpopulation at risk for early intervention. This study also found that high patient-reported distress is associated with higher odds of acute health-care services utilization in terms of ED visits and hospitalizations. Expounding the underlying mechanisms behind the association between distress and health-care utilization will provide directions on appropriate interventions in the framework of managing distress to reduce unnecessary health-care use and eventually health-care costs. Clinically, this study also adds to the body of evidence for routine distress screening and supportive care by affirming the potential cost-benefit of allocating health-care resources to this area of service. Distress screening can possibly be a useful patient-reported outcome measure that can have actionable interventions with practical benefits for health-care systems.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S1478951522001444.
Acknowledgments
We would like to thank the research co-ordinators of the Accessible Cancer Care to Enable Support for Survivors (ACCESS) program for the data collection and Michelle Tan for her assistance with data extraction and data cleaning.
Conflict of interest
None declared.